Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic and lectin domains in neuraminidase A from Streptococcus pneumoniae are capable of an intermolecular assembly: Implications for biofilm formation
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-27 , DOI: 10.1111/febs.15610 Yana Sharapova 1, 2 , Vytas Švedas 1, 2 , Dmitry Suplatov 2
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-27 , DOI: 10.1111/febs.15610 Yana Sharapova 1, 2 , Vytas Švedas 1, 2 , Dmitry Suplatov 2
Affiliation
|
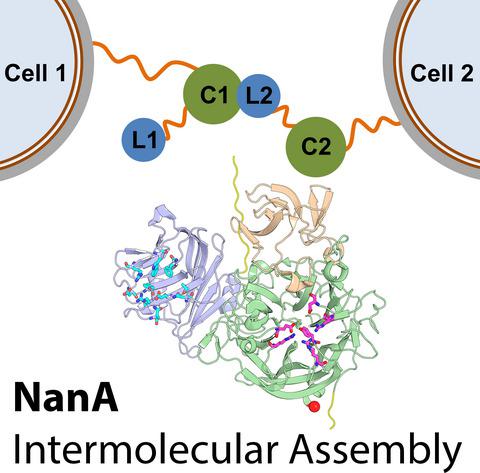
Neuraminidase A from Streptococcus pneumoniae (NanA) is a cell wall‐bound modular enzyme containing one lectin and one catalytic domain. Unlike homologous NanB and NanC expressed by the same bacterium, the two domains within one NanA molecule do not form a stable interaction and are spatially separated by a 16‐amino acid‐long flexible linker. In this work, the ability of NanA to form intermolecular assemblies was characterized using the methods of molecular modeling and bioinformatic analysis based on crystallographic data and by bringing together previously published experimental data. It was concluded that two catalytic domains, as well as one catalytic and one lectin domain, originating from two cell wall‐bound NanA molecules, can interact through a previously uncharacterized interdomain interface to form complexes stabilized by a network of intermolecular hydrogen bonds and salt bridges. Supercomputer modeling strongly indicated that artocarpin, an earlier experimentally discovered inhibitor of the pneumococcal biofilm formation, is able to bind to a site located in the catalytic domain of one NanA entity and prevent its interaction with the lectin or catalytic domain of another NanA entity, thus directly precluding the generation of intermolecular assemblies. The revealed structural adaptation is discussed as one plausible mechanism of noncatalytic participation of this potentially key pathogenicity enzyme in pneumococcal biofilm formation.
中文翻译:

肺炎链球菌神经氨酸酶A中的催化和凝集素结构域能够分子间组装:对生物膜形成的影响
肺炎链球菌的神经氨酸酶A(NanA)是一种细胞壁结合型模块化酶,包含一种凝集素和一个催化域。与同一细菌表达的同源NanB和NanC不同,一个NanA分子内的两个结构域无法形成稳定的相互作用,并且在空间上被16个氨基酸长的柔性接头隔开。在这项工作中,NanA形成分子间组装体的能力使用基于结晶学数据的分子建模和生物信息学分析方法以及先前发表的实验数据进行了表征。结论是,源自两个细胞壁结合的NanA分子的两个催化结构域以及一个催化结构域和一个凝集素结构域可以通过以前未表征的域间界面相互作用,形成通过分子间氢键和盐桥网络稳定的络合物。超级计算机模型强烈表明,阿波卡丁是一种较早的实验发现的肺炎球菌生物膜形成抑制剂,能够与位于一个NanA实体催化域中的位点结合,并阻止其与凝集素或另一个NanA实体的催化域相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。能够结合位于一个NanA实体的催化结构域中的位点并阻止其与另一NanA实体的凝集素或催化结构域的相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。能够结合位于一个NanA实体的催化结构域中的位点并阻止其与另一NanA实体的凝集素或催化结构域的相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。
更新日期:2020-10-27
中文翻译:

肺炎链球菌神经氨酸酶A中的催化和凝集素结构域能够分子间组装:对生物膜形成的影响
肺炎链球菌的神经氨酸酶A(NanA)是一种细胞壁结合型模块化酶,包含一种凝集素和一个催化域。与同一细菌表达的同源NanB和NanC不同,一个NanA分子内的两个结构域无法形成稳定的相互作用,并且在空间上被16个氨基酸长的柔性接头隔开。在这项工作中,NanA形成分子间组装体的能力使用基于结晶学数据的分子建模和生物信息学分析方法以及先前发表的实验数据进行了表征。结论是,源自两个细胞壁结合的NanA分子的两个催化结构域以及一个催化结构域和一个凝集素结构域可以通过以前未表征的域间界面相互作用,形成通过分子间氢键和盐桥网络稳定的络合物。超级计算机模型强烈表明,阿波卡丁是一种较早的实验发现的肺炎球菌生物膜形成抑制剂,能够与位于一个NanA实体催化域中的位点结合,并阻止其与凝集素或另一个NanA实体的催化域相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。能够结合位于一个NanA实体的催化结构域中的位点并阻止其与另一NanA实体的凝集素或催化结构域的相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。能够结合位于一个NanA实体的催化结构域中的位点并阻止其与另一NanA实体的凝集素或催化结构域的相互作用,因此直接排除了分子间组装的产生。讨论揭示的结构适应性是这种潜在的关键致病性酶在肺炎球菌生物膜形成中非催化参与的一种可能的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号