当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A single‐step synthesis of 1,3,4,6‐tetraaryl‐5‐aryliminopiperazin‐2‐one
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-10-30 , DOI: 10.1002/jhet.4179 Moustafa E. El‐Araby 1 , Abdelsattar M. E. Omar 1, 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-10-30 , DOI: 10.1002/jhet.4179 Moustafa E. El‐Araby 1 , Abdelsattar M. E. Omar 1, 2
Affiliation
|
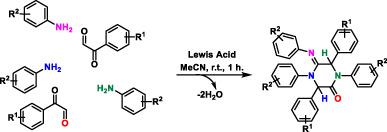
Chemical diversity is a strong driver in drug discovery, and chemical reactions that produce multi‐substituted heterocycles can result in significant chemical diversity. Here, we report a reaction that leads to highly substituted piperazinone derivatives in a single step. This was discovered accidently while reacting phenylglyoxal and anilines under Povarov conditions. We elucidated the structure of the compound formed when 2‐(4‐trifluoromethyphenyl)‐2‐oxoacetaldehyde was reacted with 4‐methoxyaniline as (1,4‐bis(4‐hydroxyphenyl)‐5‐[(4‐hydroxyphenyl)imino]‐3,6‐diphenylpiperazin‐2‐one) (5e). The structure was confirmed after extensive spectral analyses, including correlation spectroscopy, nuclear Overhauser effect spectroscopy, heteronuclear single quantum correlation spectroscopy, and heteronuclear multiple‐bond correlation nuclear magnetic resonance spectroscopy, in addition to high‐resolution mass spectrometry. This reaction is unprecedented and can potentially be exploited as a short route to highly diversified heterocycles.
中文翻译:

1,3,4,6-四芳基-5-芳基哌嗪-2-酮的一步合成
化学多样性是药物发现的重要推动力,而产生多取代杂环的化学反应会导致巨大的化学多样性。在这里,我们报告了一个反应,该反应可一步完成高度取代的哌嗪酮衍生物。这是在Povarov条件下使苯乙二醛和苯胺反应时意外发现的。我们阐明了当2-(4-三氟甲基苯基)-2-氧乙醛与4-甲氧基苯胺反应时形成的化合物的结构为(1,4-双(4-羟基苯基)-5-[[(4-羟基苯基)亚氨基]- 3,6-二苯基哌嗪-2-酮)(5e)。结构经过广泛的光谱分析,包括相关质谱法,核Overhauser效应光谱法,异核单量子相关光谱法和异核多键相关核磁共振光谱法,以及高分辨率质谱法,得到了确认。该反应是空前的,有可能被用作开发高度多样化杂环的捷径。
更新日期:2020-10-30
中文翻译:

1,3,4,6-四芳基-5-芳基哌嗪-2-酮的一步合成
化学多样性是药物发现的重要推动力,而产生多取代杂环的化学反应会导致巨大的化学多样性。在这里,我们报告了一个反应,该反应可一步完成高度取代的哌嗪酮衍生物。这是在Povarov条件下使苯乙二醛和苯胺反应时意外发现的。我们阐明了当2-(4-三氟甲基苯基)-2-氧乙醛与4-甲氧基苯胺反应时形成的化合物的结构为(1,4-双(4-羟基苯基)-5-[[(4-羟基苯基)亚氨基]- 3,6-二苯基哌嗪-2-酮)(5e)。结构经过广泛的光谱分析,包括相关质谱法,核Overhauser效应光谱法,异核单量子相关光谱法和异核多键相关核磁共振光谱法,以及高分辨率质谱法,得到了确认。该反应是空前的,有可能被用作开发高度多样化杂环的捷径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号