当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple plasmid origin‐of‐transfer regions might aid the spread of antimicrobial resistance to human pathogens
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/mbo3.1129 Jan Zrimec 1
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/mbo3.1129 Jan Zrimec 1
Affiliation
|
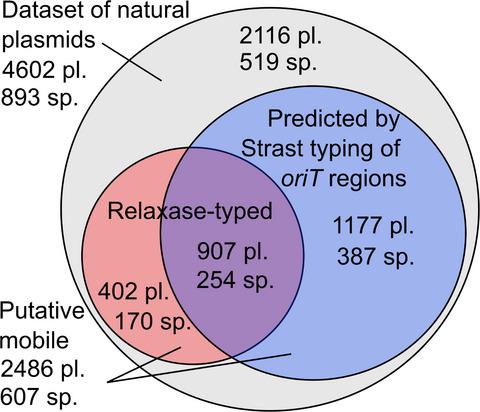
Antimicrobial resistance poses a great danger to humanity, in part due to the widespread horizontal gene transfer of plasmids via conjugation. Modeling of plasmid transfer is essential to uncovering the fundamentals of resistance transfer and for the development of predictive measures to limit the spread of resistance. However, a major limitation in the current understanding of plasmids is the incomplete characterization of the conjugative DNA transfer mechanisms, which conceals the actual potential for plasmid transfer in nature. Here, we consider that the plasmid‐borne origin‐of‐transfer substrates encode specific DNA structural properties that can facilitate finding these regions in large datasets and develop a DNA structure‐based alignment procedure for typing the transfer substrates that outperforms sequence‐based approaches. Thousands of putative DNA transfer substrates are identified, showing that plasmid mobility can be twofold higher and span almost twofold more host species than is currently known. Over half of all putative mobile plasmids contain the means for mobilization by conjugation systems belonging to different mobility groups, which can hypothetically link previously confined host ranges across ecological habitats into a robust plasmid transfer network. This hypothetical network is found to facilitate the transfer of antimicrobial resistance from environmental genetic reservoirs to human pathogens, which might be an important driver of the observed rapid resistance development in humans and thus an important point of focus for future prevention measures.
中文翻译:

多个质粒来源的转移区域可能有助于对人类病原体的抗菌素耐药性的传播
抗微生物药物耐药性对人类构成巨大危险,部分原因是质粒通过缀合进行广泛的水平基因转移。质粒转移建模对于揭示耐药性转移的基本原理和制定预测措施以限制耐药性传播至关重要。然而,目前对质粒理解的一个主要限制是结合 DNA 转移机制的不完整特征,这掩盖了自然界中质粒转移的实际潜力。在这里,我们认为质粒携带的转移底物起源编码特定的 DNA 结构特性,可以促进在大型数据集中找到这些区域,并开发基于 DNA 结构的比对程序来分型转移底物,其性能优于基于序列的方法。鉴定了数以千计的假定 DNA 转移底物,表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。
更新日期:2020-12-23
中文翻译:

多个质粒来源的转移区域可能有助于对人类病原体的抗菌素耐药性的传播
抗微生物药物耐药性对人类构成巨大危险,部分原因是质粒通过缀合进行广泛的水平基因转移。质粒转移建模对于揭示耐药性转移的基本原理和制定预测措施以限制耐药性传播至关重要。然而,目前对质粒理解的一个主要限制是结合 DNA 转移机制的不完整特征,这掩盖了自然界中质粒转移的实际潜力。在这里,我们认为质粒携带的转移底物起源编码特定的 DNA 结构特性,可以促进在大型数据集中找到这些区域,并开发基于 DNA 结构的比对程序来分型转移底物,其性能优于基于序列的方法。鉴定了数以千计的假定 DNA 转移底物,表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。表明与目前已知的相比,质粒迁移率可以高两倍,并且跨越几乎两倍的宿主物种。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。超过一半的假定移动质粒包含通过属于不同移动组的接合系统进行移动的手段,这可以假设将先前限制的跨生态栖息地的宿主范围连接到一个强大的质粒转移网络中。发现这个假设网络有助于将抗菌素耐药性从环境遗传库转移到人类病原体,这可能是观察到的人类耐药性快速发展的重要驱动因素,因此也是未来预防措施的重点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号