当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CuO{001} as the Most Active Exposed Facet for Allylic Oxidation of Cyclohexene via a Greener Route
ChemCatChem ( IF 3.8 ) Pub Date : 2020-10-30 , DOI: 10.1002/cctc.202001645 Diptangshu Datta Mal 1 , Joyjit Kundu 1 , Debabrata Pradhan 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-10-30 , DOI: 10.1002/cctc.202001645 Diptangshu Datta Mal 1 , Joyjit Kundu 1 , Debabrata Pradhan 1
Affiliation
|
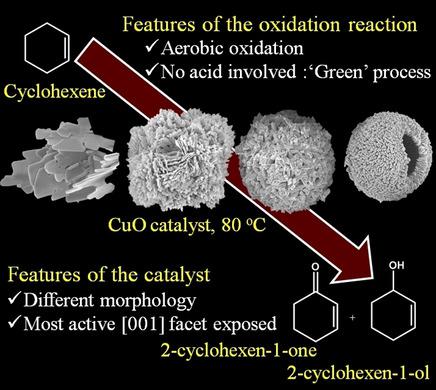
Allylic oxidation of olefins to α,β‐unsaturated carbonyl compounds, especially2‐cyclohexen‐1‐one, by the oxidation of cyclohexene is a prime chemical transformation in the organic industry due to its immense application as an intermediate in the synthesis of fine chemicals, pharmaceutical products, and perfumery products. Herein, we demonstrate microstructured copper oxide (CuO) as an efficient catalyst to oxidize cyclohexene to 2‐cyclohexen‐1‐oneand other value‐added intermediates in the presence of aerobic molecular oxygen as oxidant at 80 °C with outstanding conversion of cyclohexene and high selectivity towards 2‐cyclohexen‐1‐one. CuO with diverse shapes was synthesized by a one‐step hydrothermal method. CuO plates (PLs) and flowers (FLs) were obtained by varying the ammonia and sodium hydroxide concentration, and CuO spheres (SPs) and hollow spheres (HSs) were prepared by varying the urea concentration. Under optimized conditions, the CuO FL2 catalyst shows the highest conversion of cyclohexene (97.6 %) due to its smaller size, higher surface charge, and high‐energy {001} exposed facets. Reaction parameters such as reaction temperature, reaction duration, and catalyst concentration were varied to obtain the optimal reaction conditions for cyclohexene oxidation. Moreover, the CuO FL2 catalyst was recycled several times without any significant loss of catalytic activity, which ascertains recyclability and high stability of the catalyst for industrial use.
中文翻译:

CuO {001}是通过绿色路线对环己烯进行烯丙基氧化的最活跃暴露面
通过环己烯的氧化将烯烃烯丙氧化为α,β-不饱和羰基化合物,尤其是2-环己烯-1-酮是有机工业中的主要化学转化,这是因为其在精细化学品合成中的大量应用,药品和香水产品。本文中,我们证明了微结构化氧化铜(CuO)是一种有效的催化剂,可在80°C的需氧分子氧作为氧化剂存在下,将环己烯氧化为2-环己烯-1-酮和其他增值中间体,并具有出色的环己烯转化率和高对2-环己烯-1-酮的选择性。通过一步水热法合成了各种形状的CuO。通过改变氨和氢氧化钠的浓度获得CuO板(PLs)和花朵(FLs),并通过改变尿素浓度来制备CuO球(SPs)和空心球(HSs)。在优化的条件下,CuO FL2催化剂由于其较小的尺寸,较高的表面电荷和高能{001}暴露的小平面而显示出最高的环己烯转化率(97.6%)。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。较高的表面电荷和高能{001}暴露的小平面。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。较高的表面电荷和高能{001}暴露的小平面。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。
更新日期:2020-10-30
中文翻译:

CuO {001}是通过绿色路线对环己烯进行烯丙基氧化的最活跃暴露面
通过环己烯的氧化将烯烃烯丙氧化为α,β-不饱和羰基化合物,尤其是2-环己烯-1-酮是有机工业中的主要化学转化,这是因为其在精细化学品合成中的大量应用,药品和香水产品。本文中,我们证明了微结构化氧化铜(CuO)是一种有效的催化剂,可在80°C的需氧分子氧作为氧化剂存在下,将环己烯氧化为2-环己烯-1-酮和其他增值中间体,并具有出色的环己烯转化率和高对2-环己烯-1-酮的选择性。通过一步水热法合成了各种形状的CuO。通过改变氨和氢氧化钠的浓度获得CuO板(PLs)和花朵(FLs),并通过改变尿素浓度来制备CuO球(SPs)和空心球(HSs)。在优化的条件下,CuO FL2催化剂由于其较小的尺寸,较高的表面电荷和高能{001}暴露的小平面而显示出最高的环己烯转化率(97.6%)。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。较高的表面电荷和高能{001}暴露的小平面。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。较高的表面电荷和高能{001}暴露的小平面。改变反应参数,例如反应温度,反应持续时间和催化剂浓度,以获得用于环己烯氧化的最佳反应条件。而且,CuO FL2催化剂被循环使用了几次,而催化活性没有任何明显的损失,这确定了工业用催化剂的可循环性和高稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号