Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-10-24 , DOI: 10.1016/j.jcmgh.2020.10.010 Kyo Sasaki 1 , Sohji Nishina 1 , Akira Yamauchi 2 , Kotaro Fukuda 3 , Yuichi Hara 1 , Masahiro Yamamura 4 , Kensuke Egashira 5 , Keisuke Hino 1
|
Background & Aims
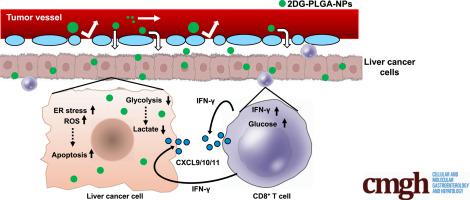
Immune checkpoint inhibitors have shed light on the importance of antitumor immunity as a therapeutic strategy for hepatocellular carcinoma (HCC). The altered glucose metabolism known as the Warburg effect recently has gained attention as a cancer immune-resistance mechanism. Considering glycolysis inhibitors as therapeutic agents, their specific delivery to cancer cells is critical not to induce adverse effects. Thus, we investigated antitumor effects of a glycolysis inhibitor, consisting of 2-deoxy-D-glucose (2DG)-encapsulated poly(lactic-co-glycolic acid) (PLGA) nanoparticles (2DG-PLGA-NPs), against hepatocellular carcinoma in mice.
Methods
The antitumor effects of 2DG-PLGA-NPs were examined using hepatoma cell lines, xenograft tumors, and hepatocarcinogenic and syngeneic mouse models.
Results
The 2DG-PLGA-NPs induced cytotoxic effects and antitumor immunity through enhanced T-cell trafficking. In addition, 2DG-PLGA-NPs induced decreased lactate production and increased interferon-γ–positive T cells in liver tumors. Human CD8+ T cells cocultured with 2DG-PLGA-NP–treated Huh7 cells showed their increased interferon-γ production and glucose uptake compared with the CD8+ T cells co-cultured with PLGA-NP–treated Huh7 cells. Chemotaxis of CD8+ T cells was suppressed by lactate and enhanced by glucose. Interferon-γ enhanced CD8+ T-cell chemotaxis in both an autocrine and paracrine manner. Notably, the 2DG-PLGA-NPs augmented chemokine (CXCL9/CXCL10) production in liver tumors via interferon-γ–Janus kinase–signal transducers and activator of transcription pathway and 5' adenosine monophosphate-activated protein kinase–mediated suppression of histone H3 lysine 27 trimethylation. These 2DG-PLGA-NPs not only amplified antitumor effects induced by sorafenib or an anti–programmed death-1 antibody, but also suppressed anti–programmed death-1–resistant tumors.
Conclusions
The newly developed 2DG-PLGA-NPs showed antitumor immunity and cytotoxicity in liver tumors in mice, suggesting the potential of 2DG-PLGA-NPs for future clinical applications.
中文翻译:

纳米颗粒介导的 2-脱氧-D-葡萄糖递送诱导小鼠肝肿瘤的抗肿瘤免疫和细胞毒性
背景与目标
免疫检查点抑制剂阐明了抗肿瘤免疫作为肝细胞癌 (HCC) 治疗策略的重要性。被称为 Warburg 效应的葡萄糖代谢改变最近作为一种癌症免疫抵抗机制受到了关注。将糖酵解抑制剂视为治疗剂,它们向癌细胞的特异性递送对于不引起不良反应至关重要。因此,我们研究了糖酵解抑制剂的抗肿瘤作用,该抑制剂由 2-脱氧-D-葡萄糖(2DG)包裹的聚(乳酸-乙醇酸共聚物)(PLGA)纳米颗粒(2DG-PLGA-NPs)组成,在老鼠。
方法
2DG-PLGA-NPs 的抗肿瘤作用使用肝癌细胞系、异种移植肿瘤、肝癌和同基因小鼠模型进行了检查。
结果
2DG-PLGA-NPs 通过增强 T 细胞运输诱导细胞毒性作用和抗肿瘤免疫。此外,2DG-PLGA-NPs 诱导肝肿瘤中乳酸产生减少和干扰素-γ 阳性 T 细胞增加。与与 PLGA-NP 处理的 Huh7细胞共培养的 CD8 + T 细胞相比,与 2DG-PLGA-NP 处理的 Huh7 细胞共培养的人 CD8 + T 细胞显示出增加的干扰素-γ 产生和葡萄糖摄取。CD8 + T 细胞的趋化性被乳酸抑制并被葡萄糖增强。干扰素-γ 增强 CD8 +自分泌和旁分泌两种方式的 T 细胞趋化性。值得注意的是,2DG-PLGA-NPs 通过干扰素-γ-Janus 激酶-信号转导和转录通路激活剂和 5' 腺苷单磷酸激活蛋白激酶-介导的组蛋白 H3 赖氨酸抑制增加了肝脏肿瘤中趋化因子(CXCL9/CXCL10)的产生27 三甲基化。这些 2DG-PLGA-NPs 不仅增强了索拉非尼或抗程序性死亡 1 抗体诱导的抗肿瘤作用,而且抑制了抗程序性死亡 1 抗性肿瘤。
结论
新开发的 2DG-PLGA-NPs 在小鼠肝脏肿瘤中显示出抗肿瘤免疫和细胞毒性,表明 2DG-PLGA-NPs 在未来的临床应用中具有潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号