Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2020-10-23 , DOI: 10.1016/j.jcmgh.2020.10.011 Shengmin Yan 1 , Bilon Khambu 1 , Xiaoyun Chen 2 , Zheng Dong 3 , Grace Guo 4 , Xiao-Ming Yin 1
|
Background & Aims
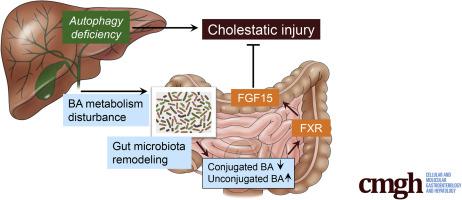
The functions of the liver and the intestine are closely tied in both physiological and pathologic conditions. The gut microbiota (GM) often cause deleterious effects during hepatic pathogenesis. Autophagy is essential for liver homeostasis, but the impact of hepatic autophagy function on liver-gut interaction remains unknown. Here we investigated the effect of hepatic autophagy deficiency (Atg5Δhep) on GM and in turn the effect of GM on the liver pathology.
Methods
Fecal microbiota were analyzed by 16S sequencing. Antibiotics were used to modulate GM. Cholestyramine was used to reduce the enterohepatic bile acid (BA) level. The functional role of fibroblast growth factor 15 (FGF15) and ileal farnesoid X receptor (FXR) was examined in mice overexpressing FGF15 gene or in mice given a fibroblast growth factor receptor-4 (FGFR4) inhibitor.
Results
Atg5Δhep causes liver injury and alterations of intestinal BA composition, with a lower proportion of tauro-conjugated BAs and a higher proportion of unconjugated BAs. The composition of GM is significantly changed with an increase in BA-metabolizing bacteria, leading to an increased expression of ileal FGF15 driven by FXR that has a higher affinity to unconjugated BAs. Notably, antibiotics or cholestyramine treatment decreased FGF15 expression and exacerbated liver injury. Consistently, inhibition of FGF15 signaling in the liver enhances liver injury.
Conclusions
Deficiency of autophagy function in the liver can affect intestinal environment, leading to gut dysbiosis. Surprisingly, such changes provide an adaptive protection against the liver injury through the FGF15-FGFR4 signaling. Antibiotics use in the condition of liver injury may thus have unexpected adverse consequences via the gut-liver axis.
中文翻译:

肝自噬缺陷通过 FGF15-FGFR4 信号重塑肠道微生物群以实现适应性保护
背景与目标
肝脏和肠道的功能在生理和病理条件下都密切相关。肠道微生物群 (GM) 经常在肝脏发病过程中造成有害影响。自噬对于肝脏稳态至关重要,但肝脏自噬功能对肝肠相互作用的影响仍不清楚。在这里,我们研究了肝脏自噬缺陷 (Atg5Δhep) 对 GM 的影响,进而研究了 GM 对肝脏病理学的影响。
方法
通过 16S 测序分析粪便微生物群。抗生素用于调节 GM。消胆胺用于降低肠肝胆汁酸 (BA) 水平。在过表达 FGF15 基因的小鼠或给予成纤维细胞生长因子受体 4 (FGFR4) 抑制剂的小鼠中检查了成纤维细胞生长因子 15 (FGF15) 和回肠法尼醇 X 受体 (FXR) 的功能作用。
结果
Atg5Δhep 导致肝损伤和肠道 BA 组成的改变,其中 tauro 结合 BA 的比例较低,未结合的 BA 比例较高。GM 的组成随着 BA 代谢细菌的增加而发生显着变化,导致 FXR 驱动的回肠 FGF15 表达增加,对未结合的 BA 具有更高的亲和力。值得注意的是,抗生素或消胆胺治疗降低了 FGF15 的表达并加剧了肝损伤。一致地,抑制肝脏中的 FGF15 信号传导会增强肝损伤。
结论
肝脏自噬功能不足会影响肠道环境,导致肠道生态失调。令人惊讶的是,这些变化通过 FGF15-FGFR4 信号传导提供了针对肝损伤的适应性保护。因此,在肝损伤情况下使用抗生素可能会通过肠-肝轴产生意想不到的不良后果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号