当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of functionalized allenes from tartaric acid
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-28 , DOI: 10.1016/j.tet.2020.131706 Kodambahalli S. Shruthi , Piyal Singh , Kavirayani R. Prasad
中文翻译:

由酒石酸立体选择性合成官能化的烯
更新日期:2020-11-21
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-28 , DOI: 10.1016/j.tet.2020.131706 Kodambahalli S. Shruthi , Piyal Singh , Kavirayani R. Prasad
|
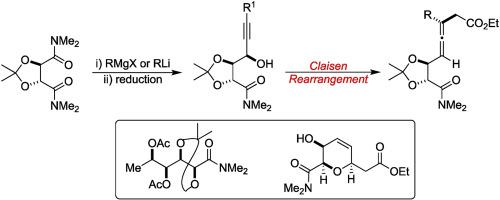
Desymmetrization of the C2-symmetric bis-dimethylamide derived from tartaric acid by controlled addition of alkynyl lithium reagents furnished the mono alkynyl ketones which on reduction produced the corresponding propargyl alcohols with good diastereoselectivity. The propargyl alcohols were transformed to allenes using Claisen rearrangement. The formed allenes serve as a precursor for the synthesis of one of the THP units of the natural product sorangicin and for the synthesis of the polyhydroxy unit present in natural product anamarine.
中文翻译:

由酒石酸立体选择性合成官能化的烯
通过控制地添加炔基锂试剂对酒石酸衍生的C 2对称的双二甲基酰胺进行不对称化处理,得到了单炔基酮,还原后生成了具有良好非对映选择性的相应炔丙基醇。使用克莱森重排将炔丙醇转化为丙二烯。形成的异位烯用作天然产物茄红素的THP单元之一的合成和天然产物阿那莫林中存在的多羟基单元的合成的前体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号