当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-phase parallel synthesis of 1,3-thiazole library adorned with dipeptidyl chains
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-24 , DOI: 10.1016/j.tet.2020.131702 Min-Jeong Cha , Aizhan Abdildinova , Young-Dae Gong
中文翻译:

二肽基链修饰的1,3-噻唑文库的固相平行合成
更新日期:2020-11-21
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-24 , DOI: 10.1016/j.tet.2020.131702 Min-Jeong Cha , Aizhan Abdildinova , Young-Dae Gong
|
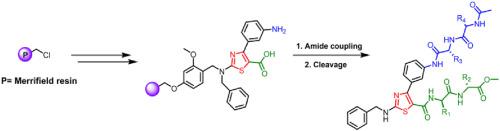
In this study, we report a solid-phase synthesis of 1,3-thiazole based peptidomimetic molecules. The key reaction step is dehydrative cyclization of thiourea resin intermediate with 2-bromo-1-(3-nitrophenyl)ethanone to afford 1,3-thiazole core with benzyl-nitro group attached. Further modification of the nitro group with peptide elongations yielded resin-bound N-benzyl-1,3-thiazole derivatives with short peptide chains at C4 and C5 positions. Cleavage from the resin delivered compounds in moderate yields. Synthesized compounds had high purities (>97%).
中文翻译:

二肽基链修饰的1,3-噻唑文库的固相平行合成
在这项研究中,我们报告了基于1,3-噻唑的拟肽分子的固相合成。关键的反应步骤是将硫脲树脂中间体与2-溴-1-(3-硝基苯基)乙酮进行脱水环化反应,制得带有苄基硝基的1,3-噻唑核。用肽延伸物进一步修饰硝基,得到在C4和C5位置具有短肽链的树脂结合的N-苄基-1,3-噻唑衍生物。从树脂上切下以中等产率递送化合物。合成的化合物具有高纯度(> 97%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号