Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-21 , DOI: 10.1016/j.tet.2020.131696 Nanaji Yerramsetti , Lavanya Dampanaboina , Venugopal Mendu , Satyanarayana Battula
|
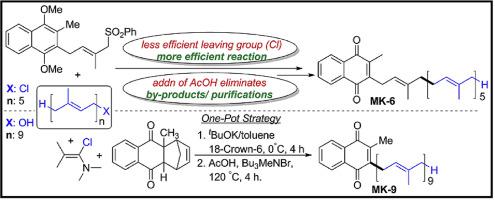
Here we report a practical and efficient method for the synthesis of menaquinone vitamin (K2) analog MK-6 in all trans forms through “1 + 5 convergent synthetic approach” of pentaprenyl chloride with monoprenyl menadione derivative. In the synergistic factors, less efficient leaving group/more efficient nucleophile (Cl) in the substrate makes it more prominent reaction by eliminating all Sn2’ side reaction products. Further, the addition of acetic acid in the last step (desulfonation) of reaction sequence removes the limitations of the reactions in terms of cyclized side product (multiple reactions of pentaprenyl alcohol with Et3B), byproduct (Et3B, incendiary compound) formations and their interruption in the tricky purification processes. The utility of this method was further extended to find an efficient one-pot synthesis to MK-9 to the gram scale synthesis. This approach is economical and efficient and avoids the awkward chromatographic separation processes.
中文翻译:

协同因素在甲萘醌[K 2 ]类似物MK-6的合成中具有很高的便利性:为访问MK-9的高效一锅式协议的应用
在这里,我们报告了通过戊戊烯基氯与单异戊烯基甲萘醌衍生物的“ 1 + 5会聚合成方法”,以所有反式合成甲萘醌维生素(K 2)类似物MK-6的实用有效方法。在协同作用因素中,底物中较低效率的离去基团/较有效的亲核试剂(Cl)通过消除所有S n 2'副反应产物而使其反应更加突出。此外,在反应顺序的最后一步(脱硫)中添加乙酸,消除了环化副产物(戊戊烯醇与Et 3 B的多次反应),副产物(Et 3)方面的反应限制。B,燃烧化合物)的形成及其在棘手的纯化过程中的中断。进一步扩展了该方法的实用性,以找到从MK-9到克级合成的有效一锅合成。这种方法经济高效,并且避免了笨拙的色谱分离过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号