Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-21 , DOI: 10.1016/j.tet.2020.131678 Xiong-Wei Liu , Jing Yue , Zheng Li , Dan Wu , Min-Yi Tian , Qi-Lin Wang , Ying Zhou
|
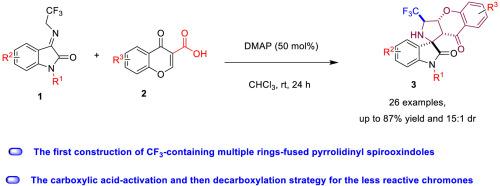
Inspired by the chemistry and biology of fluorine-containing molecules, chromanones and pyrrolidinyl spirooxindoles, herein we report a highly diastereoselective [3 + 2] cycloaddition reaction of commercially available 3-carboxylic acid chromones and N-2,2,2-trifluoroethylisatin ketimines as azomethine ylide precursors in the presence of the catalyst DMAP, which enabled diversity-oriented synthesis of a series of potentially bioactive scaffolds containing CF3, chromanone and pyrrolidinyl spirooxindole with high efficiency (up to 87% yield and 15:1 diastereomeric ratio). The high efficiency of the cycloaddition relies on the use of a carboxylic acid-activation and then decarboxylation strategy for the less reactive chromones. In particular, this is the first construction of CF3-containing multiple rings-fused pyrrolidinyl spirooxindoles, bearing four contiguous stereogenic centers including one tetra substituted carbon, which might be valuable in medicinal chemistry.
中文翻译:

DMAP催化的脱羧[3 + 2]环加成反应:非对映选择性合成三氟甲基化苯并二氢吡喃并并吡咯烷基螺环辛多酯的策略
受含氟分子,发色团和吡咯烷基螺氧基辛酯的化学和生物学影响,本文报道了市售的3-羧酸色酮和N-2,2,2-三氟乙基异丁酮酮的非对映选择性[3 + 2]环加成反应,催化剂DMAP存在下的甲亚胺叶立德前体,可实现一系列含有CF 3的潜在生物活性支架的多样性导向合成,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃,苯并二氢吡喃酮,苯并二氢吡喃酮和吡咯烷基吡咯并吲哚(效率高达87%和15:1非对映异构体比例)环加成反应的高效率取决于对反应性较低的色酮的羧酸活化和脱羧策略的使用。特别是,这是第一个包含CF3的多环稠合吡咯烷基螺旋螺内酯结构,带有四个连续的立体异构中心,包括一个四取代碳,这在医学化学中可能是有价值的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号