当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal and photochemical analysis of bimetallic Bi-Mo and Bi-W carbonyl complexes
Solid State Sciences ( IF 3.4 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.solidstatesciences.2020.106451 Sumit Saha , Jenn M. Amador , Sara Goberna-Ferron , Shawn R. Decker , Deok-Hie Park , Douglas A. Keszler
Solid State Sciences ( IF 3.4 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.solidstatesciences.2020.106451 Sumit Saha , Jenn M. Amador , Sara Goberna-Ferron , Shawn R. Decker , Deok-Hie Park , Douglas A. Keszler
|
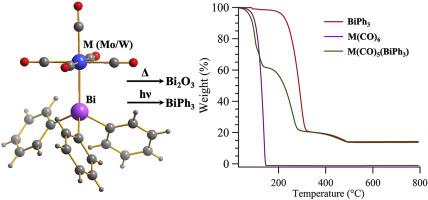
Abstract We investigated the thermal behavior of bimetallic Bi-Mo and Bi-W carbonyl complexes, [Mo(CO)5(BiPh3)] and [W(CO)5(BiPh3)] respectively, to understand the mechanism of decomposition. TGA-DTA, TGA-MS, FTIR, powder XRD and EDX were used to analyze these compounds at different temperatures. At elevated temperature (∼150 °C) loss of the M(CO)6 (M = Mo, W) group occurs first, followed by the loss of phenyl groups at ∼300 °C, resulting in the formation of yellow Bi2O3 in presence of air. Additionally, we studied the photochemical behavior of these bimetallic Bi-Mo and Bi-W carbonyl complexes. UV irradiation of these compounds caused the loss of the metal carbonyl, resulting in the formation of BiPh3.
中文翻译:

双金属 Bi-Mo 和 Bi-W 羰基配合物的热和光化学分析
摘要 我们分别研究了双金属 Bi-Mo 和 Bi-W 羰基配合物 [Mo(CO)5(BiPh3)] 和 [W(CO)5(BiPh3)] 的热行为,以了解分解机制。TGA-DTA、TGA-MS、FTIR、粉末 XRD 和 EDX 用于在不同温度下分析这些化合物。在升高的温度 (~150 °C) 下,首先发生 M(CO)6 (M = Mo, W) 基团的损失,然后在~300 °C 时失去苯基,导致在存在下形成黄色的 Bi2O3空气。此外,我们研究了这些双金属 Bi-Mo 和 Bi-W 羰基配合物的光化学行为。这些化合物的紫外线照射导致金属羰基的损失,导致形成 BiPh3。
更新日期:2021-03-01
中文翻译:

双金属 Bi-Mo 和 Bi-W 羰基配合物的热和光化学分析
摘要 我们分别研究了双金属 Bi-Mo 和 Bi-W 羰基配合物 [Mo(CO)5(BiPh3)] 和 [W(CO)5(BiPh3)] 的热行为,以了解分解机制。TGA-DTA、TGA-MS、FTIR、粉末 XRD 和 EDX 用于在不同温度下分析这些化合物。在升高的温度 (~150 °C) 下,首先发生 M(CO)6 (M = Mo, W) 基团的损失,然后在~300 °C 时失去苯基,导致在存在下形成黄色的 Bi2O3空气。此外,我们研究了这些双金属 Bi-Mo 和 Bi-W 羰基配合物的光化学行为。这些化合物的紫外线照射导致金属羰基的损失,导致形成 BiPh3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号