Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-10-27 , DOI: 10.1016/j.jmb.2020.10.027 Meenakumari Muthuramalingam 1 , Sean K Whittier 1 , Scott Lovell 2 , Kevin P Battaile 3 , Shoichi Tachiyama 1 , David K Johnson 4 , Wendy L Picking 1 , William D Picking 1
|
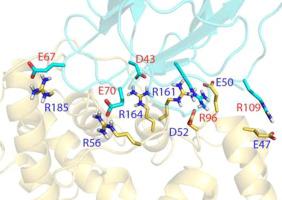
Many Gram-negative bacterial pathogens use type III secretion systems (T3SS) to inject proteins into eukaryotic cells to subvert normal cellular functions. The T3SS apparatus (injectisome) shares a common architecture in all systems studied thus far, comprising three major components – the cytoplasmic sorting platform, envelope-spanning basal body and external needle with tip complex. The sorting platform consists of an ATPase (SctN) connected to “pods” (SctQ) having six-fold symmetry via radial spokes (SctL). These pods interface with the 24-fold symmetric SctD inner membrane ring (IR) via an adaptor protein (SctK). Here we report the first high-resolution structure of a SctK protein family member, PscK from Pseudomonas aeruginosa, as well as the structure of its interacting partner, the cytoplasmic domain of PscD (SctD). The cytoplasmic domain of PscD forms a forkhead-associated (FHA) fold, like that of its homologues from other T3SS. PscK, on the other hand, forms a helix-rich structure that does not resemble any known protein fold. Based on these structural findings, we present the first model for an interaction between proteins from the sorting platform and the IR. We also test the importance of the PscD residues predicted to mediate this electrostatic interaction using a two-hybrid analysis. The functional need for these residues in vivo was then confirmed by monitoring secretion of the effector ExoU. These structures will contribute to the development of atomic-resolution models of the entire sorting platform and to our understanding of the mechanistic interface between the sorting platform and the basal body of the injectisome.
中文翻译:

铜绿假单胞菌SctK和SctD的结构揭示了III型分泌系统基体和分选平台的界面
许多革兰氏阴性细菌病原体使用 III 型分泌系统 (T3SS) 将蛋白质注入真核细胞中,以破坏正常的细胞功能。T3SS 装置(注射体)在迄今为止研究的所有系统中具有共同的架构,包括三个主要组件 - 细胞质分选平台、跨越包膜的基体和带有尖端复合体的外部针。该分选平台由一个 ATPase (SctN) 组成,该 ATPase (SctN)通过径向辐条 ( SctL) 连接到具有六重对称性的“豆荚”( SctQ)。这些豆荚通过衔接蛋白 (SctK)与 24 重对称 SctD 内膜环 (IR) 连接。在这里,我们报告了 SctK 蛋白家族成员(来自铜绿假单胞菌的 PscK)的第一个高分辨率结构,以及其相互作用伙伴 PscD 的细胞质结构域 (SctD) 的结构。PscD 的胞质结构域形成叉头相关 (FHA) 折叠,就像其他 T3SS 的同源物一样。另一方面,PscK 形成富含螺旋的结构,与任何已知的蛋白质折叠都不相似。基于这些结构发现,我们提出了分选平台和 IR 蛋白质之间相互作用的第一个模型。我们还使用双杂交分析测试了预测介导这种静电相互作用的 PscD 残基的重要性。然后通过监测效应器 ExoU 的分泌来证实体内对这些残基的功能需求。这些结构将有助于开发整个分选平台的原子分辨率模型,并有助于我们理解分选平台和注射体基体之间的机械界面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号