Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.yjmcc.2020.10.010 Nian Wang 1 , Heng Ma 2 , Jing Li 2 , ChaoYang Meng 2 , Jiang Zou 2 , Hao Wang 2 , Ke Liu 2 , Meidong Liu 2 , Xianzhong Xiao 2 , Huali Zhang 2 , Kangkai Wang 2
|
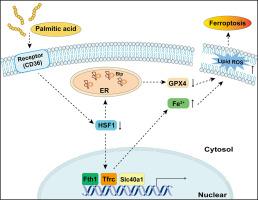
Palmitic acid (PA)-induced myocardial injury is considered a critical contributor to the development of obesity and type 2 diabetes mellitus (T2DM)-related cardiomyopathy. However, the underlying mechanism has not been fully understood. Here, we demonstrated that PA induced the cell death of H9c2 cardiomyoblasts in a dose- and time-dependent manner, while different ferroptosis inhibitors significantly abrogated the cell death of H9c2 cardiomyoblasts and primary neonatal rat cardiomyocytes exposed to PA. Mechanistically, PA decreased the protein expression levels of both heat shock factor 1 (HSF1) and glutathione peroxidase 4 (GPX4) in a dose- and time-dependent manner, which were restored by different ferroptosis inhibitors. Overexpression of HSF1 not only alleviated PA-induced cell death and lipid peroxidation but also improved disturbed iron homeostasis by regulating the transcription of iron metabolism-related genes (e.g., Fth1, Tfrc, Slc40a1). Additionally, PA-blocked GPX4 protein expression was evidently restored by HSF1 overexpression. Inhibition of endoplasmic reticulum (ER) stress rather than autophagy contributed to HSF1-mediated GPX4 expression. Moreover, GPX4 overexpression protected against PA-induced ferroptosis, whereas knockdown of GPX4 reversed the anti-ferroptotic effect of HSF1. Consistent with the in vitro findings, PA-challenged Hsf1−/− mice exhibited more serious ferroptosis, increased Slc40a1 and Fth1 mRNA expression, decreased GPX4 and TFRC expression and enhanced ER stress in the heart compared with Hsf1+/+ mice. Altogether, HSF1 may function as a key defender against PA-induced ferroptosis in cardiomyocytes by maintaining cellular iron homeostasis and GPX4 expression.
中文翻译:

HSF1 作为心肌细胞中棕榈酸诱导的铁死亡的关键防御者
棕榈酸 (PA) 引起的心肌损伤被认为是肥胖和 2 型糖尿病 (T2DM) 相关心肌病发生的关键因素。然而,其根本机制尚未被完全理解。在此,我们证明PA以剂量和时间依赖性方式诱导H9c2成肌细胞的细胞死亡,而不同的铁死亡抑制剂显着消除暴露于PA的H9c2成肌细胞和原代新生大鼠心肌细胞的细胞死亡。从机制上讲,PA 以剂量和时间依赖性方式降低热休克因子 1 (HSF1) 和谷胱甘肽过氧化物酶 4 (GPX4)的蛋白表达水平,而不同的铁死亡抑制剂可以恢复这种水平。 HSF1的过表达不仅减轻了PA诱导的细胞死亡和脂质过氧化,而且还通过调节铁代谢相关基因(例如Fth1 、 Tfrc 、 Slc40a1 )的转录改善了紊乱的铁稳态。此外,HSF1 过表达明显恢复了 PA 阻断的 GPX4 蛋白表达。内质网 (ER) 应激的抑制而非自噬促进了 HSF1 介导的 GPX4 表达。此外,GPX4 过表达可以防止 PA 诱导的铁死亡,而 GPX4 的敲低则逆转了 HSF1 的抗铁死亡作用。与体外研究结果一致,与Hsf 1 +/+小鼠相比,PA 攻击的Hsf 1 −/−小鼠表现出更严重的铁死亡、 Slc40a1和Fth1 mRNA 表达增加、GPX4 和 TFRC 表达减少以及心脏内质网应激增强。 总而言之,HSF1 可能通过维持细胞铁稳态和 GPX4 表达,充当心肌细胞中 PA 诱导的铁死亡的关键防御者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号