当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced photocatalytic reaction and mechanism for treating cyanide-containing wastewater by silicon-based nano-titania
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105512 Yang Zhang , Yali Zhang , Yaoguo Huang , Xia Chen , Hongyou Cui , Ming Wang
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105512 Yang Zhang , Yali Zhang , Yaoguo Huang , Xia Chen , Hongyou Cui , Ming Wang
|
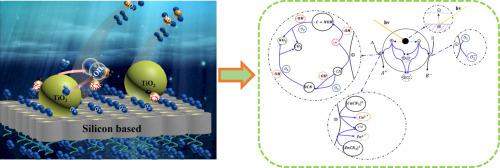
Abstract The treatment of cyanide-containing wastewater is very important for the sustainable development of gold industry. Treating wastewater by nano-titania (nano-TiO2) has the advantage of adsorbing heavy metals and catalyzing the decomposition of cyanogen simultaneously. Since the catalysis is limited by solid-liquid diphase system, the photocatalytic and adsorption efficiency is relatively low. This study aimed to enhance catalytic process based on silicon-based nano-TiO2 composite functional material to treat deeply cyanide-containing wastewater. The characteristics of the silicon-based nano-TiO2 material were studied by Brunner−Emmet−Teller (BET) and field emission scanning electron microscopy (FESEM)/ energy dispersive spectrometer (EDS), which indicated that the material has excellent dispersibility and large specific surface area(146.6±2.4 m2·g−1). Further, the comparison of the treatment effects of cyanide wastewater with pure nano-TiO2 and silicon-based nano-TiO2 material demonstrates that the latter is more effective on cyanide degradation. Almost 99.8% of cyanide degradation was achieved by silicon-based nano-TiO2 material under light irradiation within 135 min. The mechanism and X-ray photoelectron spectroscopy (XPS) analysis. It was found that the final decompositions of Copper cyanide complex and zinc cyanide complex are CO2, NO2, Cu2+ and Zn2+.
中文翻译:

硅基纳米二氧化钛处理含氰废水的增强光催化反应及机理
摘要 含氰废水的处理对黄金工业的可持续发展具有重要意义。纳米二氧化钛(nano-TiO2)处理废水具有吸附重金属和同时催化氰分解的优点。由于催化受到固液二相体系的限制,光催化和吸附效率较低。本研究旨在增强基于硅基纳米TiO2复合功能材料的催化工艺处理深度含氰废水。通过Brunner-Emmet-Teller(BET)和场发射扫描电子显微镜(FESEM)/能谱仪(EDS)研究了硅基纳米TiO2材料的特性,表明该材料具有优异的分散性和大的比值。表面积(146。6±2.4 m2·g-1)。此外,纯纳米二氧化钛和硅基纳米二氧化钛材料处理氰化物废水的效果比较表明,后者对氰化物降解更有效。硅基纳米 TiO2 材料在 135 分钟内在光照射下实现了近 99.8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。硅基纳米二氧化钛材料在光照射下 135 分钟内实现了 8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。硅基纳米二氧化钛材料在光照射下 135 分钟内实现了 8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。
更新日期:2020-12-01
中文翻译:

硅基纳米二氧化钛处理含氰废水的增强光催化反应及机理
摘要 含氰废水的处理对黄金工业的可持续发展具有重要意义。纳米二氧化钛(nano-TiO2)处理废水具有吸附重金属和同时催化氰分解的优点。由于催化受到固液二相体系的限制,光催化和吸附效率较低。本研究旨在增强基于硅基纳米TiO2复合功能材料的催化工艺处理深度含氰废水。通过Brunner-Emmet-Teller(BET)和场发射扫描电子显微镜(FESEM)/能谱仪(EDS)研究了硅基纳米TiO2材料的特性,表明该材料具有优异的分散性和大的比值。表面积(146。6±2.4 m2·g-1)。此外,纯纳米二氧化钛和硅基纳米二氧化钛材料处理氰化物废水的效果比较表明,后者对氰化物降解更有效。硅基纳米 TiO2 材料在 135 分钟内在光照射下实现了近 99.8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。硅基纳米二氧化钛材料在光照射下 135 分钟内实现了 8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。硅基纳米二氧化钛材料在光照射下 135 分钟内实现了 8% 的氰化物降解。机理和X射线光电子能谱(XPS)分析。发现氰化铜络合物和氰化锌络合物的最终分解物是CO2、NO2、Cu2+和Zn2+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号