European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-10-23 , DOI: 10.1016/j.ejmech.2020.112956 Marc-Antoine Bazin , Sandrine Cojean , Fabrice Pagniez , Guillaume Bernadat , Christian Cavé , Isabelle Ourliac-Garnier , Marie-Renée Nourrisson , Cathy Morgado , Carine Picot , Olivier Leclercq , Blandine Baratte , Thomas Robert , Gérald F. Späth , Najma Rachidi , Stéphane Bach , Philippe M. Loiseau , Patrice Le Pape , Pascal Marchand
|
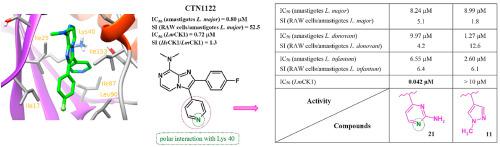
Leishmaniasis constitutes a severe public health problem, with an estimated prevalence of 12 million cases. This potentially fatal disease has a worldwide distribution and in 2012, the fatal Visceral Leishmaniasis (VL) was declared as new emerging disease in Europe, mainly due to global warming, with expected important public health impact. The available treatments are toxic, costly or lead to parasite resistance, thus there is an urgent need for new drugs with new mechanism of action. Previously, we reported the discovery of CTN1122, a potent imidazo[1,2-a]pyrazine-based antileishmanial hit compound targeting L-CK1.2 at low micromolar ranges. Here, we described structurally related, safe and selective compounds endowed with antiparasitic properties, better than miltefosine, the reference therapy by oral route. L-CK1.2 homology model gave the first structural explanations of the role of 4-pyridyl (CTN1122) and 2-aminopyrimidin-4-yl (compound 21) moieties, at the position 3 of the central core, in the low micromolar to nanomolar L-CK1.2 inhibition, whereas N-methylpyrazole derivative 11 remained inactive against the parasite kinase.
中文翻译:

咪唑并[1,2 - a ]吡啶类抗疟药的体外鉴定及对大酪蛋白激酶1抑制作用的评价
利什曼病是一个严重的公共卫生问题,估计患病率为1200万例。这种潜在的致命疾病在全球范围内分布,并且在2012年,致命的内脏利什曼病(VL)被宣布为欧洲的一种新兴疾病,主要是由于全球气候变暖以及对公众健康的重大影响。可用的治疗是有毒的,昂贵的或导致寄生虫抗性,因此迫切需要具有新作用机理的新药物。此前,我们报道的发现CTN1122,一个强有力的咪唑并[1,2一在低微摩尔范围内靶向L-CK1.2的基于吡嗪的抗疟疾抗击化合物。在这里,我们描述了具有抗寄生虫特性的结构相关,安全和选择性的化合物,优于口服途径的参考疗法miltefosine。L-CK1.2同源性模型给出了在低微摩尔浓度下,在中心核心位置3处的4-吡啶基(CTN1122)和2-氨基嘧啶-4-基(化合物21)部分的作用的第一个结构解释。纳摩尔L-CK1.2抑制,而N-甲基吡唑衍生物11仍然对寄生虫激酶无活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号