European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.ejmech.2020.112959 Qiuyue Zhang , Xuexuan Wu , Jianrui Zhou , Lixiao Zhang , Xiaoli Xu , Lianshan Zhang , Qidong You , Lei Wang
|
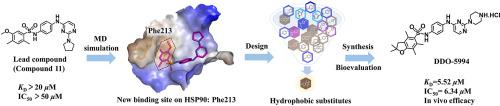
HSP90-CDC37 protein-protein interaction (PPI) works as a kinase specific-molecular chaperone system to regulate the maturation of kinases. Currently, selectively disrupting HSP90-CDC37 PPI, rather than the direct inhibition of the ATPase function of HSP90, is emerging as a promising strategy for cancer therapy by specifically blocking the maturation of kinases. However, due to the limited understanding of HSP90-CDC37 binding interface, design of small molecule inhibitors targeting HSP90-CDC37 PPI is challenging. In this work, based on the binding mode of compound 11 (previously reported by our group), we discovered a hydrophobic pocket centered on Phe213, which was previously unknown, contributing to the binding affinity of HSP90-CDC37 PPI inhibitors. A series of hydrophobic substituted inhibitors were utilized to confirm the importance of Phe213 hydrophobic core. Finally, we obtained an optimum compound DDO-5994 (exhibited an ideal binding pattern on hydrophobic core) with improved binding affinity (KD = 5.52 μM) and antiproliferative activity (IC50 = 6.34 μM). Both in vitro and in vivo assays confirmed DDO-5994 as a promising inhibitor to exhibit ideal antitumor efficacy through blocking HSP90-CDC37 PPI.
中文翻译:

基于疏水核心的针对HSP90-CDC37蛋白质-蛋白质相互作用的抑制剂的设计,合成和生物评价
HSP90-CDC37蛋白-蛋白相互作用(PPI)作为激酶特异性分子伴侣系统来调节激酶的成熟。当前,选择性地破坏HSP90-CDC37 PPI,而不是直接抑制HSP90的ATPase功能,正在通过特异性地阻断激酶的成熟而成为一种有前景的癌症治疗策略。但是,由于对HSP90-CDC37结合界面的了解有限,靶向HSP90-CDC37 PPI的小分子抑制剂的设计具有挑战性。在这项工作中,基于化合物11的结合方式(我们小组先前的报道),我们发现了一个以Phe213为中心的疏水口袋,该口袋以前是未知的,有助于形成HSP90-CDC37 PPI抑制剂的结合亲和力。利用一系列疏水取代的抑制剂来确认Phe213疏水核心的重要性。最后,我们得到的最佳化合物DDO-5994具有改善的结合亲和力((显示对疏水核的理想结合模式)ķ d = 5.52 μ M)和抗增殖活性(IC 50 = 6.34 μ M)。二者在体外和体内测定法证实DDO-5994 通过阻断HSP90-CDC37 PPI发挥理想的抗肿瘤作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号