Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-10-28 , DOI: 10.1016/j.ensm.2020.10.022 Aiping Wang , Zheyi Zou , Da Wang , Yue Liu , Yajie Li , Junming Wu , Maxim Avdeev , Siqi Shi
|
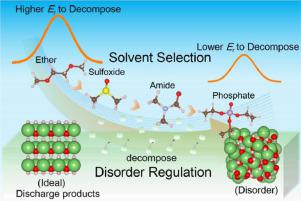
Redox mediators are promised to thermodynamically resolve the cathode irreversibility of Li-air battery. However, the sluggish chemical reaction between mediators and discharge products severely restrains fast charging. Here, we combine ab initio calculations and machine learning method to investigate the reaction kinetics between LiOH and I2, and demonstrate the critical role of the disorder degree of LiOH and the solvent effect. The Li+ desorption is identified as the rate determining step (rds) of the reaction. While LiOH turns from the crystalline to disordered/amorphous structure, the rds energy barrier will be reduced by ∼500 meV. The functional group of the solvent is detected as the key to regulating the solvation effect and phosphate-based solvent is predicted to accelerate the decomposition kinetics most with the strongest solvation capability. These findings indicate that the faster reaction kinetics between mediators and the discharge products can be achieved by rational discharge product structure regulation and appropriate solvent selection.
中文翻译:

通过从头算和机器学习识别影响锂空气电池反应动力学的化学因素
氧化还原介体有望在热力学上解决锂空气电池的阴极不可逆性。但是,介体和放电产物之间的缓慢化学反应严重限制了快速充电。在这里,我们结合了从头算和机器学习方法来研究LiOH和I 2之间的反应动力学,并证明了LiOH的无序度和溶剂效应的关键作用。Li +解吸被确定为反应的速率确定步骤(rds)。而将LiOH从结晶转变为无序/非晶结构中,RDS势垒将减少约500 meV。检测到溶剂的官能团是调节溶剂化效果的关键,并且预测磷酸盐基溶剂会以最强的溶剂化能力最大程度地加速分解动力学。这些发现表明,通过合理的放电产物结构调节和适当的溶剂选择,可以实现介体和放电产物之间更快的反应动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号