当前位置:
X-MOL 学术
›
Diam. Relat. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The origin of color of 1330 nm center diamonds
Diamond and Related Materials ( IF 4.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.diamond.2020.108151 Thomas Hainschwang , Franck Notari , Gianna Pamies
Diamond and Related Materials ( IF 4.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.diamond.2020.108151 Thomas Hainschwang , Franck Notari , Gianna Pamies
|
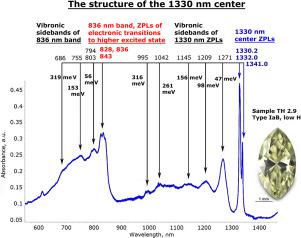
Abstract This study covers hydrogen-rich fancy color diamonds that exhibit complex spectra from the UV all the way to the mid-IR. The diamonds with such spectra that are included here show a large range of colors from brownish yellow to brown, yellow-green to olive and gray to violet. The color origin of such diamonds has always been stated as “hydrogen-related”, without much evidence pointing towards hydrogen actually causing absorptions in the visible spectral range, but only based on their unusually high IR active hydrogen content determined via their FTIR spectra. The diamonds analyzed during this work always showed a series of absorptions in the near-infrared at 7495, 7850, 8255, and 8615 cm−1. For the first time, this here presented study shows the results of low temperature near-infrared spectroscopy performed for a series of differently colored diamonds that all showed these NIR absorptions. When measured at 77 K, it became clear that these NIR bands are actually part of an electronic optical center with ZPLs at 1329.8 to 1330.2 nm (7520–7518 cm−1)/1331.8 to 1332.2 nm (7508–7506 cm−1) and 1341 to 1341.2 nm (7457–7456 cm−1). In this paper we will refer to this defect as the “1330 nm center” (which corresponds to 7519 cm−1) for the sake of brevity. The detailed analysis of the spectra has demonstrated that the colors of diamonds that exhibit the 1330 nm center spectra are caused partially by this same center, and by complex absorption bands associated to two series of ZPLs represented by a number of sharp bands between 965 and 1001 nm, referred to as the 990 nm series in this study. Of these, the 990 nm series was found only in diamonds with significant IR active hydrogen concentrations, while the 1330 nm center was determined to be independent from the concentration of IR active hydrogen. The 1330 nm center was found in spectra lacking the 990 nm series of ZPLs, but the 990 nm series has never been found in spectra without the 1330 nm center. We are suggesting that the defects involved in these absorptions are all nickel‑nitrogen-related, with the 1330 nm center lacking hydrogen while it seems reasonable to assume that the 990 nm series includes hydrogen in its structure.
中文翻译:

1330 nm 主钻的颜色起源
摘要 这项研究涵盖了富氢彩色钻石,这些钻石在从紫外线到中红外线的范围内表现出复杂的光谱。此处包含的具有此类光谱的钻石显示出从棕黄色到棕色、黄绿色到橄榄色以及灰色到紫色等多种颜色。此类钻石的颜色起源一直被称为“与氢有关”,没有太多证据表明氢实际上会导致可见光谱范围内的吸收,但仅基于通过 FTIR 光谱确定的异常高的 IR 活性氢含量。在这项工作中分析的钻石总是在 7495、7850、8255 和 8615 cm-1 处显示出一系列近红外吸收。首次,此处介绍的这项研究显示了对一系列不同颜色的钻石进行的低温近红外光谱分析结果,这些钻石均显示出这些 NIR 吸收。在 77 K 下测量时,很明显这些 NIR 波段实际上是电子光学中心的一部分,ZPL 位于 1329.8 至 1330.2 nm(7520-7518 cm-1)/1331.8 至 1332.2 nm(7508-7506 cm-1)和1341 至 1341.2 nm (7457–7456 cm-1)。在本文中,为简洁起见,我们将此缺陷称为“1330 nm 中心”(对应于 7519 cm-1)。光谱的详细分析表明,显示 1330 nm 中心光谱的钻石的颜色部分是由同一中心引起的,以及与两个系列 ZPL 相关的复杂吸收带,由 965 和 1001 之间的许多锐带表示纳米,在本研究中称为 990 nm 系列。其中,990 nm 系列仅在具有显着 IR 活性氢浓度的钻石中发现,而 1330 nm 中心被确定为与 IR 活性氢浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。而 1330 nm 中心被确定与红外活性氢的浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。而 1330 nm 中心被确定与红外活性氢的浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。
更新日期:2020-12-01
中文翻译:

1330 nm 主钻的颜色起源
摘要 这项研究涵盖了富氢彩色钻石,这些钻石在从紫外线到中红外线的范围内表现出复杂的光谱。此处包含的具有此类光谱的钻石显示出从棕黄色到棕色、黄绿色到橄榄色以及灰色到紫色等多种颜色。此类钻石的颜色起源一直被称为“与氢有关”,没有太多证据表明氢实际上会导致可见光谱范围内的吸收,但仅基于通过 FTIR 光谱确定的异常高的 IR 活性氢含量。在这项工作中分析的钻石总是在 7495、7850、8255 和 8615 cm-1 处显示出一系列近红外吸收。首次,此处介绍的这项研究显示了对一系列不同颜色的钻石进行的低温近红外光谱分析结果,这些钻石均显示出这些 NIR 吸收。在 77 K 下测量时,很明显这些 NIR 波段实际上是电子光学中心的一部分,ZPL 位于 1329.8 至 1330.2 nm(7520-7518 cm-1)/1331.8 至 1332.2 nm(7508-7506 cm-1)和1341 至 1341.2 nm (7457–7456 cm-1)。在本文中,为简洁起见,我们将此缺陷称为“1330 nm 中心”(对应于 7519 cm-1)。光谱的详细分析表明,显示 1330 nm 中心光谱的钻石的颜色部分是由同一中心引起的,以及与两个系列 ZPL 相关的复杂吸收带,由 965 和 1001 之间的许多锐带表示纳米,在本研究中称为 990 nm 系列。其中,990 nm 系列仅在具有显着 IR 活性氢浓度的钻石中发现,而 1330 nm 中心被确定为与 IR 活性氢浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。而 1330 nm 中心被确定与红外活性氢的浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。而 1330 nm 中心被确定与红外活性氢的浓度无关。在缺少 990 nm 系列 ZPL 的光谱中发现了 1330 nm 中心,但在没有 1330 nm 中心的光谱中从未发现过 990 nm 系列。我们认为这些吸收中涉及的缺陷都与镍氮相关,1330 nm 中心缺乏氢,而假设 990 nm 系列在其结构中包含氢似乎是合理的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号