Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-10-28 , DOI: 10.1016/j.cej.2020.127531 Yu Cheng , Haoran Guo , Pengfei Yuan , Xinpan Li , Lirong Zheng , Rui Song
|
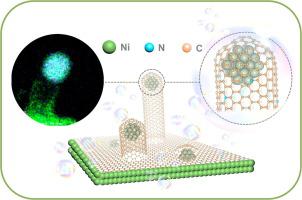
To satisfy the practical requirements of electrochemical water splitting, developing a flexible, effective and sustainable approach for scalable production of bifunctional electrocatalyst for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is a tremendous issue to be rationally addressed. Herein, a solid-state diffusion strategy with Ni foam and N-doped carbon layers is applied to prepare multi-interfacial, self-supported and N-doped carbon nanotube (NCNT) encapsulated Ni nanoparticles (NPs) array bifunctional electrocatalyst (NCNT-NP@NF). Prominently, this NCNT-NP@NF is scalable to meet the application requirements and can be used as a binder-free electrode for direct the water splitting. The optimal sample demonstrate outstanding HER/OER performance in 1.0 M KOH, with low overpotential (η10) at 10 mA cm−2 during HER (96.1 mV) and OER (240 mV) process, which is extremely superior to the most reported metal-based electrocatalysts and even better than commercial IrO2 (400 mV at 10 mA cm−2 for OER). Strikingly, when these bifunctional electrocatalysts are employed in a dual-electrode electrolyzer for water splitting, the NCNT-NP@NF only needs cell voltage of 1.54 V to drive overall water splitting in 1.0 M KOH, and displays excellent long-term stability (150 h for water splitting). Moreover, experiments combined with density functional theory (DFT) calculations clarify that the remarkable electrochemical activity and stability are mainly attribute to the synergistic effect of the uniform distribution of Ni NPs, heteroatomic doping, as well as district confinement effect of NCNT leading to enhanced electronic multi-interfacial transmission, all these factors co-accelerate electrocatalytic kinetics. Accordingly, this solid-state diffusion method possesses a favorable potential to construct a multi-interfacial and self-supported electrocatalysts in the fields of sustainable supply of clean energies.
中文翻译:

Ni纳米颗粒包裹在垂直N掺杂碳纳米管中的自支撑双功能电催化剂,用于有效的总水分解
为了满足电化学水分解的实际要求,开发一种灵活,有效和可持续的方法,以用于氢气释放反应(HER)和氧气释放反应(OER)的可扩展生产双功能电催化剂是需要合理解决的巨大问题。在本文中,采用具有Ni泡沫和N掺杂碳层的固态扩散策略来制备多界面,自支撑和N掺杂碳纳米管(NCNT)封装的Ni纳米颗粒(NPs)阵列双功能电催化剂(NCNT-NP)。 @NF)。突出的是,该NCNT-NP @ NF具有可扩展性,可以满足应用需求,可以用作直接水分解的无粘合剂电极。最佳样品在1.0 M KOH中表现出出色的HER / OER性能,且低电势(η10)在HER(96.1 mV)和OER(240 mV)过程中在10 mA cm -2时,这要优于大多数报道的金属基电催化剂,甚至优于商用IrO 2(在10 mA cm -2时为400 mV)对于OER)。令人惊讶的是,当将这些双功能电催化剂用于双电极电解水中进行水分解时,NCNT-NP @ NF仅需要1.54 V的电池电压来驱动1.0 M KOH中的总水分解,并显示出出色的长期稳定性(150 h用于水分解)。此外,结合密度泛函理论(DFT)计算的实验表明,出色的电化学活性和稳定性主要归因于镍纳米颗粒的均匀分布,杂原子掺杂以及NCNT的区域限制效应的协同效应,从而增强了电子性能。多界面传输,所有这些因素共同加速了电催化动力学。因此,









































 京公网安备 11010802027423号
京公网安备 11010802027423号