Cell Systems ( IF 9.0 ) Pub Date : 2020-10-27 , DOI: 10.1016/j.cels.2020.10.001 Surya Pandey 1 , Adam Gruenbaum 1 , Tamara Kanashova 2 , Philipp Mertins 2 , Philippe Cluzel 3 , Nicolas Chevrier 1
|
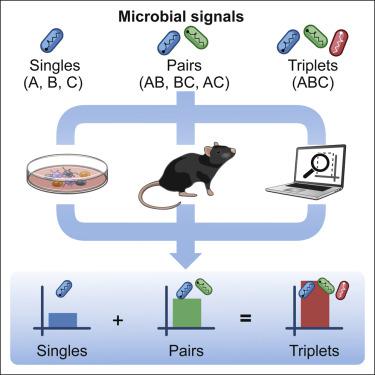
The immune system makes decisions in response to combinations of multiple microbial inputs. We do not understand the combinatorial logic governing how higher-order combinations of microbial signals shape immune responses. Here, using coculture experiments and statistical analyses, we discover a general property for the combinatorial sensing of microbial signals, whereby the effects of triplet combinations of microbial signals on immune responses can be predicted by combining the effects of single and pairs. Mechanistically, we find that singles and pairs dictate the information signaled by triplets in mouse and human DCs at the levels of transcription, chromatin, and protein secretion. We exploit this simplifying property to develop cell-based immunotherapies prepared with adjuvant combinations that trigger protective responses in mouse models of cancer. We conclude that the processing of multiple input signals by innate immune cells is governed by pairwise effects, which will inform the rationale combination of adjuvants to manipulate immunity.
中文翻译:

病原体感应途径的成对刺激预测对多佐剂组合的免疫反应
免疫系统根据多种微生物输入的组合做出决定。我们不了解控制微生物信号的高阶组合如何形成免疫反应的组合逻辑。在这里,使用共培养实验和统计分析,我们发现了微生物信号组合感知的一般特性,由此可以通过组合单个和成对的影响来预测微生物信号的三重组合对免疫反应的影响。从机制上讲,我们发现单身和成对决定了小鼠和人类 DCs 在转录、染色质和蛋白质分泌水平上由三联体发出的信息。我们利用这种简化的特性来开发基于细胞的免疫疗法,这些疗法采用佐剂组合制备,可在小鼠癌症模型中触发保护性反应。我们得出结论,先天免疫细胞对多个输入信号的处理受成对效应控制,这将为佐剂组合以操纵免疫提供依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号