Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2020-10-28 , DOI: 10.1016/j.xcrp.2020.100237 Shanshan Lu , Yanmei Shi , Nannan Meng , Siyu Lu , Yifu Yu , Bin Zhang
|
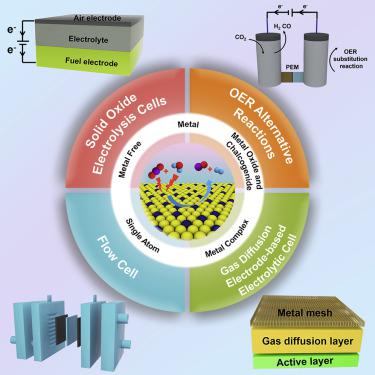
The traditional synthesis of syngas, a mixture of CO and H2, relies on the reverse water gas shift reaction at high temperature and thus consumes considerable energy and resources. In this regard, the electrochemical conversion of CO2-H2O to CO-H2 provides an emerging alternative technique to conquer these shortages. This short review highlights the recent advances and future trends in the electrocatalytic transformation of CO2 and H2O to syngas with a tunable H2:CO ratio. We summarize the latest advances in metals, metal oxides and chalcogenides, metal complex catalysts, single-atom catalysts, and metal-free catalysts with an emphasis on controlling the CO:H2 ratio, which is vital for downstream Fischer-Tropsch process synthesis. Then we introduce versatile methods to improve the production efficiency of syngas by alternative anode reactions and advanced technologies (e.g., gas diffusion electrode-based, flow, solid oxide electrolytic cells). Finally, we provide an outlook on the current challenges and promising opportunities in the field of syngas synthesis.
中文翻译:

通过CO 2和H 2 O的共还原电合成合成气
传统合成气(CO和H 2的混合物)依赖于高温下的反向水煤气变换反应,因此消耗了大量的能源和资源。在这方面,从CO 2 -H 2 O到CO-H 2的电化学转化为克服这些不足提供了一种新兴的替代技术。这篇简短的评论重点介绍了将CO 2和H 2 O电催化转化为可调H 2合成气的最新进展和未来趋势。:CO比。我们总结了金属,金属氧化物和硫族化物,金属络合物催化剂,单原子催化剂和无金属催化剂的最新进展,重点是控制CO:H 2的比例,这对于下游的费-托合成工艺至关重要。然后,我们介绍了通过替代性阳极反应和先进技术(例如,基于气体扩散电极的,流动式,固体氧化物电解槽)来提高合成气生产效率的通用方法。最后,我们对合成气合成领域的当前挑战和有前途的机遇进行了展望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号