Biochimie ( IF 3.3 ) Pub Date : 2020-10-27 , DOI: 10.1016/j.biochi.2020.10.016 Natalya M. Subbotina , Alexey M. Chernykh , Anton I. Taranov , Anna D. Shebanova , Olga V. Moiseeva , Marta Ferraroni , Marina P. Kolomytseva
|
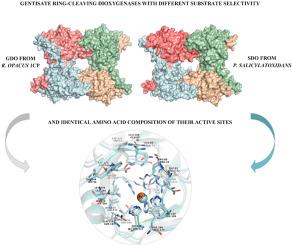
Gentisate 1,2-dioxygenases belong to the class III ring-cleaving dioxygenases catalyzing key reactions of aromatic compounds degradation by aerobic microorganisms. In the present work, the results of complete molecular, structural, and functional investigations of the gentisate 1,2-dioxygenase (rho-GDO) from a gram-positive bacterium Rhodococcus opacus 1CP growing on 3-hydroxybenzoate as a sole source of carbon and energy are presented. The purified enzyme showed a narrow substrate specificity. Among fourteen investigated substrate analogues only gentisate was oxidized by the enzyme, what can be potentially applied in biosensor technologies. The rho-GDO encoding gene was identified in the genomic DNA of the R. opacus 1CP. According to phylogenetic analysis, the rho-GDO belongs to the group of apparently most recently acquired activities in bacterial genera Rhodococcus, Arthrobacter, Corynebacterium, Nocardia, Amycolatopsis, Comamonas, and Streptomyces. Homology modeling the rho-GDO 3D-structure demonstrates the composition identity of the first-sphere residues of the active site of rho-GDO and salicylate 1,2-dioxygenase from Pseudaminobacter salicylatoxidans (RCSB PDB: 2PHD), despite of their different substrate specificities. The phenomenon described for the first time for this family of enzymes supposes a more complicated mechanism of substrate specificity than previously imagined, and makes the rho-GDO a convenient model for a novel direction of structure-function relationship studies.
中文翻译:

来自革兰氏阳性菌不透明红球菌1CP的Gentisate 1,2-双加氧酶:相同的活性位点与不同的底物选择性
Gentisate 1,2-双加氧酶属于III类环裂解双加氧酶,催化需氧微生物降解芳香族化合物的关键反应。在目前的工作中,来自革兰氏阳性细菌不透明红球菌1CP上的龙胆酸酯1,2-双加氧酶(rho - GDO)的完整分子,结构和功能研究的结果,是在3-羟基苯甲酸酯上作为唯一碳源和能量被提出。纯化的酶显示出狭窄的底物特异性。在十四种被研究的底物类似物中,只有龙胆酸酯被该酶氧化,这可能在生物传感器技术中得到应用。的RHO -GDO编码基因在基因组DNA中鉴定R.球菌1CP。根据系统发育分析,rho- GDO属于红球菌属,关节杆菌属,棒状杆菌属,诺卡氏菌,支原体,Comamonas和链霉菌属中最近获得的活动。同源性建模的RHO -GDO三维结构证明的活性位点的第一个球的残基的组合物身份RHO从-GDO和水杨酸1,2-双加氧酶Pseudaminobacter salicylatoxidans(RCSB PDB:2PHD),尽管它们的底物特异性不同。首次针对该酶家族描述的现象假设底物特异性的机制比以前想象的要复杂,并使rho- GDO成为结构-功能关系研究新方向的便捷模型。









































 京公网安备 11010802027423号
京公网安备 11010802027423号