Biochimie ( IF 3.3 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.biochi.2020.10.006 Victoria Wingert , Srijan Mukherjee , Anna J. Esser , Sidney Behringer , Segun Tanimowo , Melissa Klenzendorf , Ilia A. Derevenkov , Sergei V. Makarov , Donald W. Jacobsen , Ute Spiekerkoetter , Luciana Hannibal
|
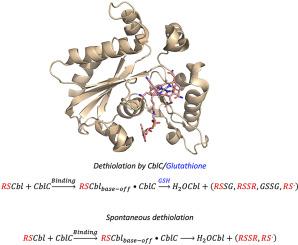
Thiolatocobalamins are a class of cobalamins comprised of naturally occurring and synthetic ligands. Glutathionylcobalamin (GSCbl) occurs naturally in mammalian cells, and also as an intermediate in the glutathione-dependent dealkylation of methylcobalamin (MeCbl) to form cob(I)alamin by pure recombinant CblC from C. elegans. Glutathione-driven deglutathionylation of GSCbl was demonstrated both in mammalian as well as in C. elegans CblC. Dethiolation is orders of magnitude faster than dealkylation of Co–C bonded cobalamins, which motivated us to investigate two synthetic thiolatocobalamins as substrates to repair the enzymatic activity of pathogenic CblC variants in humans. We report the synthesis and kinetic characterization of cysteaminylcobalamin (CyaCbl) and 2-mercaptopropionylglycinocobalamin (MpgCbl). Both CyaCbl and MpgCbl were obtained in high purity (90–95%) and yield (78–85%). UV–visible spectral properties agreed with those reported for other thiolatocobalamins with absorbance maxima observed at 372 nm and 532 nm. Both CyaCbl and MpgCbl bound to wild type human recombinant CblC inducing spectral blue-shifts characteristic of the respective base-on to base-off transitions. Addition of excess glutathione (GSH) resulted in rapid elimination of the β-ligand to give aquacobalamin (H2OCbl) as the reaction product under aerobic conditions. Further, CyaCbl and MpgCbl underwent spontaneous dethiolation thereby repairing the loss of activity of pathogenic variants of human CblC, namely R161G and R161Q. We posit that thiolatocobalamins could be exploited therapeutically for the treatment of inborn errors of metabolism that impair processing of dietary and supplemental cobalamin forms. While these disorders are targets for newborn screening in some countries, there is currently no effective treatment available to patients.
中文翻译:

硫代巴巴蛋白可修复人钴胺素加工酶CblC的致病变体活性
硫代硫辛酸是由天然和合成的配体组成的一类钴胺素。谷胱甘肽钴胺素(GSCbl)天然存在于哺乳动物细胞中,并且作为谷胱甘肽依赖的甲基钴胺素(MeCbl)脱烷基化反应的中间体,通过线虫的纯重组CblC形成Cob(I)alamin 。在哺乳动物和秀丽隐杆线虫中均证实了谷胱甘肽驱动的GSCbl脱谷胱甘肽化CblC。脱硫作用比Co-C键合的钴胺素的脱烷基作用快几个数量级,这促使我们研究了两种合成的硫代latocobalamins作为底物来修复人类病原性CblC变体的酶活性。我们报告了半胱氨酰钴胺素(CyaCbl)和2-巯基丙酰甘氨酸钴胺素(MpgCbl)的合成和动力学表征。CyaCbl和MpgCbl均以高纯度(90–95%)和收率(78–85%)获得。紫外可见光谱特性与报道的其他硫代latocobalamins的光谱特性一致,在372 nm和532 nm处观察到最大吸收。CyaCbl和MpgCbl都与野生型人类重组CblC结合,从而诱导了各自从基到基的过渡的特征性光谱蓝移。2 OCbl)作为有氧条件下的反应产物。此外,CyaCbl和MpgCbl进行自发的脱硫,从而修复了人CblC的致病变体即R161G和R161Q的活性丧失。我们假设可以将硫代latocobalamins用于治疗先天性新陈代谢错误,这些新陈代谢会损害膳食和补充钴胺素形式的加工。尽管这些疾病在某些国家是新生儿筛查的目标,但目前尚无有效的治疗方法可用于患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号