Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-10-27 , DOI: 10.1016/j.bbagen.2020.129775 Christopher J. Brown , Chandra S. Verma , David P. Lane , Dilraj Lama
|
Background
Intrinsically disordered regions (IDRs) in proteins can regulate their activity by facilitating protein-protein interactions (PPIs) as exemplified in the recruitment of the eukaryotic translation initiation factor 4E (eIF4E) protein by the protein eIF4G. Deregulation of this PPI module is central to a broad spectrum of cancer related malignancies and its targeted inhibition through bioactive peptides is a promising strategy for therapeutic intervention.
Methods
We employed molecular dynamics simulations coupled with biophysical assays to rationally develop peptide derivatives from the intrinsically disordered eIF4G scaffold by incorporating non-natural amino acids that facilitates disorder-to-order transition.
Results
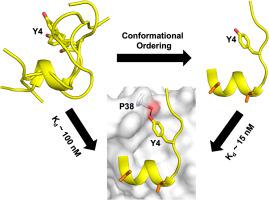
The conformational heterogeneity of these peptides and the degree of structural reorganization required to adopt the optimum mode of interaction with eIF4E underscores their differential binding affinities. The presence of a pre-structured local helical element in the ensemble of structures was instrumental in the efficient docking of the peptides on to the protein surface. The formation of Y4: P38 hydrogen-bond interaction between the peptide and eIF4E is a rate limiting event in the efficient recognition of the protein since it occurs through the disordered region of the peptide.
Conclusions
These insights were exploited to further design features into the peptide to propagate bound-state conformations in solution which resulted in the generation of a potent eIF4E binder.
General significance
The study illustrates the molecular basis of eIF4E recognition by a disordered epitope from eIF4G and its modulation to generate peptides that can potentially attenuate translation initiation in oncology.
中文翻译:

用于靶向翻译起始的内在无序的肽的构象排序
背景
蛋白质中的内在无序区域(IDR)可以通过促进蛋白质-蛋白质相互作用(PPI)来调节其活性,例如蛋白质eIF4G募集真核翻译起始因子4E(eIF4E)蛋白质就是例证。该PPI模块的失控是广泛的癌症相关恶性肿瘤的关键,其通过生物活性肽的靶向抑制是一种有希望的治疗干预策略。
方法
我们采用分子动力学模拟与生物物理分析相结合的方法,通过掺入非自然氨基酸以促进无序转移,从固有的无序eIF4G支架中合理开发出肽衍生物。
结果
这些肽的构象异质性和采用与eIF4E相互作用的最佳方式所需的结构重组程度突显了它们的不同结合亲和力。结构整体中预结构化的局部螺旋元件的存在有助于将肽有效地对接至蛋白质表面。肽和eIF4E之间形成Y4:P38氢键相互作用是有效识别蛋白质的速率限制事件,因为它是通过肽的无序区域发生的。
结论
利用这些见识进一步设计肽的功能,以在溶液中传播结合态构象,从而产生有效的eIF4E结合物。
一般意义
该研究说明了来自eIF4G的无序表位识别eIF4E的分子基础,以及对其进行调制以生成可能削弱肿瘤翻译起始的肽的调控。











































 京公网安备 11010802027423号
京公网安备 11010802027423号