Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.bbagen.2020.129770 Fiona Gordon , Saioa Elcoroaristizabal , Alan G. Ryder
|
Background
Förster Resonance Energy Transfer (FRET) is widely used to study the structure and dynamics of biomolecular systems and also causes the non-linear fluorescence response observed in multi-fluorophore proteins. Accurate FRET analysis, in terms of measuring changes in donor and acceptor spectra and energy transfer efficiency is therefore critical.
Methods
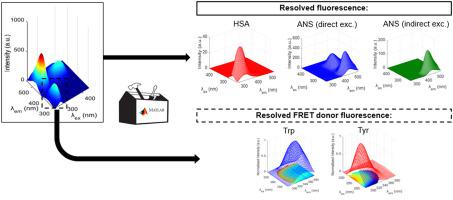
We demonstrate a novel quantitative FRET analysis using anisotropy resolved multidimensional emission spectroscopy (ARMES) in a Human Serum Albumin (HSA) and 1,8-anilinonaphathalene sulfonate (ANS) model. ARMES combines 4D measurement of polarized excitation emission matrices (pEEM) with multivariate data analysis to spectrally resolve contributing fluorophores. Multivariate analysis (Parallel Factor, PARAFAC and restricted Tucker3) was used to resolve fluorophore contributions and for modelling the quenching of HSA emission and the HSA-ANS interactions.
Results
pEEM spectra were modelled using Tucker3 which accommodates non-linearities introduced by FRET and a priori chemical knowledge was used to optimise the solution, thus resolving three components: HSA emission, ANS emission from indirect FRET excitation, and ANS emission from direct excitation. Perpendicular emission measurements were more sensitive to indirectly excited acceptor emission. PARAFAC modelling of HSA, donor emission, separated ANS FRET interacting (Tryptophan) and non-interacting (Tyrosine) components. This enabled a new way of calculating quenching constants using the multi-dimensional emission of individual donor fluorophores.
Conclusions
FRET efficiency could be calculated using the multi-dimensional, resolved emission of the interacting donor fluorophores only which yielded higher ET efficiencies compared to conventional methods.
General significance
Shows the potential of multidimensional fluorescence measurements and data analysis for more accurate FRET modelling in proteins.
中文翻译:

使用各向异性分辨多维发射光谱(ARMES)对Förster共振能量转移(FRET)建模
背景
福斯特共振能量转移(FRET)被广泛用于研究生物分子系统的结构和动力学,并且还引起多荧光团蛋白中观察到的非线性荧光反应。因此,就测量供体和受体光谱的变化以及能量转移效率而言,准确的FRET分析至关重要。
方法
我们在人类血清白蛋白(HSA)和1,8-苯胺基萘甲磺酸盐(ANS)模型中使用各向异性分辨多维发射光谱(ARMES)证明了一种新颖的定量FRET分析。ARMES将极化激发发射矩阵(pEEM)的4D测量与多元数据分析相结合,以光谱方式解析出有用的荧光团。多变量分析(平行因子,PARAFAC和限制性Tucker3)用于解析荧光团的贡献,并用于建模HSA发射的猝灭和HSA-ANS相互作用。
结果
使用适应FRET引入的非线性的Tucker3对pEEM光谱进行建模,并使用先验化学知识来优化解决方案,从而解决了三个成分:HSA发射,间接FRET激发的ANS发射和直接激发的ANS发射。垂直发射测量对间接激发的受体发射更敏感。HSA,供体发射,分离的ANS FRET相互作用(色氨酸)和非相互作用(酪氨酸)成分的PARAFAC建模。这使得能够使用单个供体荧光团的多维发射来计算猝灭常数的新方法。
结论
FRET效率只能使用相互作用的施主荧光团的多维分辨发射来计算,与传统方法相比,它产生更高的ET效率。
一般意义
显示了多维荧光测量和数据分析在蛋白质中更精确的FRET建模方面的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号