Applied Materials Today ( IF 7.2 ) Pub Date : 2020-10-27 , DOI: 10.1016/j.apmt.2020.100860 Jue Hu 1, 2 , Jacob M Miszuk 1, 2 , Kyle M Stein 1 , Hongli Sun 1, 2
|
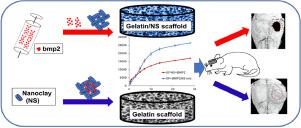
Nanoclay (Nanosilicates, NS) is appearing as an intriguing 2D nanomaterial for bone tissue engineering with multiple proposed functions, e.g., intrinsic osteoinductivity, improving mechanical properties, and drug release capacity. However, the mechanism of NS for in vivo bone regeneration has been hardly defined so far. This knowledge gap will significantly affect the design/application of NS-based biomaterials. To determine the role of NS in osteoblastic differentiation and bone formation, we used mouse calvarial-derived pre-osteoblasts (MC3T3-E1) and a clinically-relevant mouse cranial bone defect model. Instead of a hydrogel, we prepared biomimetic 3D gelatin nanofibrous scaffolds (GF) and NS-blended composite scaffolds (GF/NS) to determine the essential role of NS in critical low-dose (0.5 µg per scaffold) of BMP2-induced cranial bone regeneration. In contrast to “osteoinductivity”, our data indicated that NS could enable single-dose of BMP2, promoting significant osteoblastic differentiation while multiple-dose of BMP2 (without NS) was required to achieve similar efficacy. Moreover, our release study revealed that direct binding to NS in GF scaffolds provided stronger protection to BMP2 and sustained release compared to GF/NS composite scaffolds. Consistently, our in vivo data indicated that only BMP2/NS direct binding treatment was able to repair the large mouse cranial bone defects after 6 weeks of transplantation while neither BMP2, NS alone, nor BMP2 released from GF/NS scaffolds was sufficient to induce significant cranial bone defect repair. Therefore, we concluded that direct nanoclay-drug binding enabled sustained release is the most critical contribution to the significantly improved bone regeneration compared to other possible mechanisms based on our study.
中文翻译:

纳米粘土主要通过调节药物结合和缓释促进小鼠颅骨再生
纳米粘土(纳米硅酸盐,NS)作为一种用于骨组织工程的有趣的二维纳米材料出现,具有多种提议的功能,例如,固有的骨诱导性、改善机械性能和药物释放能力。然而,NS在体内的作用机制到目前为止,骨再生几乎没有定义。这种知识差距将显着影响基于 NS 的生物材料的设计/应用。为了确定 NS 在成骨细胞分化和骨形成中的作用,我们使用了小鼠颅骨衍生的前成骨细胞 (MC3T3-E1) 和临床相关的小鼠颅骨缺损模型。我们制备了仿生 3D 明胶纳米纤维支架 (GF) 和 NS 混合复合支架 (GF/NS),而不是水凝胶,以确定 NS 在 BMP2 诱导的颅骨的临界低剂量(0.5 µg 每个支架)中的重要作用再生。与“骨诱导性”相反,我们的数据表明 NS 可以实现单剂量的 BMP2,促进显着的成骨细胞分化,而需要多剂量的 BMP2(不含 NS)才能达到类似的功效。而且,我们的释放研究表明,与 GF/NS 复合支架相比,与 GF 支架中 NS 的直接结合为 BMP2 和持续释放提供了更强的保护。一贯地,我们的体内数据表明,只有 BMP2/NS 直接结合治疗能够在移植 6 周后修复大小鼠颅骨缺损,而单独的 BMP2、NS 和 GF/NS 支架释放的 BMP2 均不足以诱导显着的颅骨缺损修理。因此,我们得出结论,与基于我们研究的其他可能机制相比,直接纳米粘土-药物结合使持续释放成为显着改善骨再生的最关键贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号