当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Reactivity of Supported ZnO Clusters: Undercoordinated Zinc and Oxygen Atoms as Active Sites
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-10-28 , DOI: 10.1002/cphc.202000747 Xiaojuan Yu 1 , Jannik P Roth 2 , Junjun Wang 1 , Eric Sauter 1 , Alexei Nefedov 1 , Stefan Heißler 1 , Gianfranco Pacchioni 2 , Yuemin Wang 1 , Christof Wöll 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-10-28 , DOI: 10.1002/cphc.202000747 Xiaojuan Yu 1 , Jannik P Roth 2 , Junjun Wang 1 , Eric Sauter 1 , Alexei Nefedov 1 , Stefan Heißler 1 , Gianfranco Pacchioni 2 , Yuemin Wang 1 , Christof Wöll 1
Affiliation
|
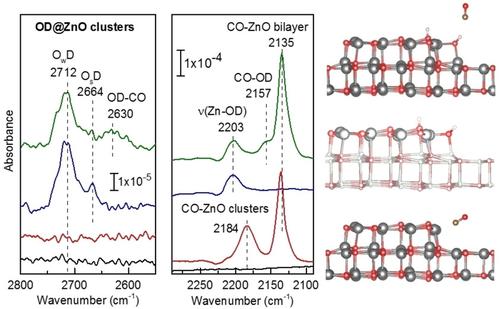
The growth of ZnO clusters supported by ZnO‐bilayers on Ag(111) and the interaction of these oxide nanostructures with water have been studied by a multi‐technique approach combining temperature‐dependent infrared reflection absorption spectroscopy (IRRAS), grazing‐emission X‐ray photoelectron spectroscopy, and density functional theory calculations. Our results reveal that the ZnO bilayers exhibiting graphite‐like structure are chemically inactive for water dissociation, whereas small ZnO clusters formed on top of these well‐defined, yet chemically passive supports show extremely high reactivity ‐ water is dissociated without an apparent activation barrier. Systematic isotopic substitution experiments using H216O/D216O/D218O allow identification of various types of acidic hydroxyl groups. We demonstrate that a reliable characterization of these OH‐species is possible via co‐adsorption of CO, which leads to a red shift of the OD frequency due to the weak interaction via hydrogen bonding. The theoretical results provide atomic‐level insight into the surface structure and chemical activity of the supported ZnO clusters and allow identification of the presence of under‐coordinated Zn and O atoms at the edges and corners of the ZnO clusters as the active sites for H2O dissociation.
中文翻译:

负载型 ZnO 团簇的化学反应性:欠配位的锌和氧原子作为活性位点
通过结合温度依赖性红外反射吸收光谱(IRRAS)、掠射发射 X-射线光电子能谱和密度泛函理论计算。我们的结果表明,表现出类石墨结构的 ZnO 双层对于水解离没有化学活性,而在这些明确定义的、化学上被动的载体顶部形成的小 ZnO 簇显示出极高的反应性——水在没有明显的活化势垒的情况下解离。使用H 2 16 O/D 2 16 O/D 2 18 O的系统同位素取代实验可以鉴定各种类型的酸性羟基。我们证明,通过 CO 的共吸附可以对这些 OH 物质进行可靠的表征,由于氢键的弱相互作用,导致 OD 频率红移。理论结果提供了对所支持的 ZnO 团簇的表面结构和化学活性的原子级洞察,并允许识别 ZnO 团簇边缘和角落处的配位不足的 Zn 和 O 原子作为 H 2 的活性位点。哦解离。
更新日期:2020-12-02
中文翻译:

负载型 ZnO 团簇的化学反应性:欠配位的锌和氧原子作为活性位点
通过结合温度依赖性红外反射吸收光谱(IRRAS)、掠射发射 X-射线光电子能谱和密度泛函理论计算。我们的结果表明,表现出类石墨结构的 ZnO 双层对于水解离没有化学活性,而在这些明确定义的、化学上被动的载体顶部形成的小 ZnO 簇显示出极高的反应性——水在没有明显的活化势垒的情况下解离。使用H 2 16 O/D 2 16 O/D 2 18 O的系统同位素取代实验可以鉴定各种类型的酸性羟基。我们证明,通过 CO 的共吸附可以对这些 OH 物质进行可靠的表征,由于氢键的弱相互作用,导致 OD 频率红移。理论结果提供了对所支持的 ZnO 团簇的表面结构和化学活性的原子级洞察,并允许识别 ZnO 团簇边缘和角落处的配位不足的 Zn 和 O 原子作为 H 2 的活性位点。哦解离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号