当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Digallane Gold Complex with a 12-Electron Auride Center: Synthesis and Computational Studies
Organometallics ( IF 2.5 ) Pub Date : 2020-10-29 , DOI: 10.1021/acs.organomet.0c00557 Kohei Susukida 1 , Leonardo I. Lugo-Fuentes 2 , Shozo Matsumae 1 , Kazuki Nakanishi 1 , Masaaki Nakamoto 1 , Yohsuke Yamamoto 1 , Rong Shang 1 , Joaquín Barroso-Flores 3, 4 , J. Oscar C. Jimenez-Halla 2
Organometallics ( IF 2.5 ) Pub Date : 2020-10-29 , DOI: 10.1021/acs.organomet.0c00557 Kohei Susukida 1 , Leonardo I. Lugo-Fuentes 2 , Shozo Matsumae 1 , Kazuki Nakanishi 1 , Masaaki Nakamoto 1 , Yohsuke Yamamoto 1 , Rong Shang 1 , Joaquín Barroso-Flores 3, 4 , J. Oscar C. Jimenez-Halla 2
Affiliation
|
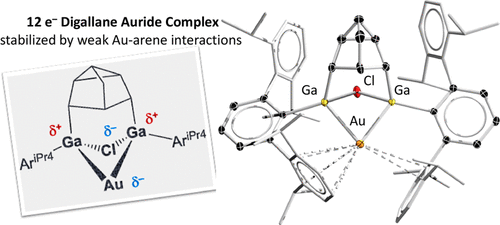
The reaction of Power’s digalladeltacyclane with Au(PPh3)Cl led to formation of a digallium gold complex (2) with both chloride and gold bridging between two gallium atoms. The bonding parameters from the solid-state structure of 2 suggested a 12e– Au center stabilized by weakly coordinated aryl groups. DFT calculations at the M05-2X/[LANL2TZ(f),6-311G(d)] level indicated a Ga–Au–Ga banana bond with no bonding interactions between gallium atoms. The gold carries an NBO charge of −0.0956 e– and is best viewed as an auride. The weak intramolecular interactions between Au p orbitals and σC–C and σC–H bonds of the adjacent aromatic rings amount to significant stabilization of the electron-deficient gold center. The digallium complex 2 represents the first structural example of a 12 e– organometallic auride complex, demonstrating its pseudohalide/hydride nature in bonding.
中文翻译:

具有12电子金化合物中心的Digallane金络合物:合成与计算研究
Power的双galladeltacyclane与Au(PPh 3)Cl的反应导致形成二镓金配合物(2),氯化物和金都桥接在两个镓原子之间。固态结构2的键合参数表明,通过弱配位的芳基可稳定12e - Au中心。在M05-2X / [LANL2TZ(f),6-311G(d)]级别的DFT计算表明,Ga-Au-Ga香蕉键与镓原子之间没有键相互作用。黄金的NBO电荷为-0.0956 e –最好被视为是硫化物。Au p轨道与σC –C和σC –H之间的弱分子内相互作用相邻芳环的键合使缺电子的金中心显着稳定。镓二配合物2代表12 e –有机金属金酰化物配合物的第一个结构实例,证明了其在键合中的假卤化物/氢化物性质。
更新日期:2020-12-14
中文翻译:

具有12电子金化合物中心的Digallane金络合物:合成与计算研究
Power的双galladeltacyclane与Au(PPh 3)Cl的反应导致形成二镓金配合物(2),氯化物和金都桥接在两个镓原子之间。固态结构2的键合参数表明,通过弱配位的芳基可稳定12e - Au中心。在M05-2X / [LANL2TZ(f),6-311G(d)]级别的DFT计算表明,Ga-Au-Ga香蕉键与镓原子之间没有键相互作用。黄金的NBO电荷为-0.0956 e –最好被视为是硫化物。Au p轨道与σC –C和σC –H之间的弱分子内相互作用相邻芳环的键合使缺电子的金中心显着稳定。镓二配合物2代表12 e –有机金属金酰化物配合物的第一个结构实例,证明了其在键合中的假卤化物/氢化物性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号