当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confinement of Ionic Liquids at Single-Ni-Sites Boost Electroreduction of CO2 in Aqueous Electrolytes
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-10-29 , DOI: 10.1021/acscatal.0c03873 Wenhao Ren 1 , Xin Tan 2 , Xianjue Chen 1 , Guobin Zhang 3 , Kangning Zhao 4 , Wanfeng Yang 1 , Chen Jia 1 , Yong Zhao 1 , Sean C. Smith 2 , Chuan Zhao 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-10-29 , DOI: 10.1021/acscatal.0c03873 Wenhao Ren 1 , Xin Tan 2 , Xianjue Chen 1 , Guobin Zhang 3 , Kangning Zhao 4 , Wanfeng Yang 1 , Chen Jia 1 , Yong Zhao 1 , Sean C. Smith 2 , Chuan Zhao 1
Affiliation
|
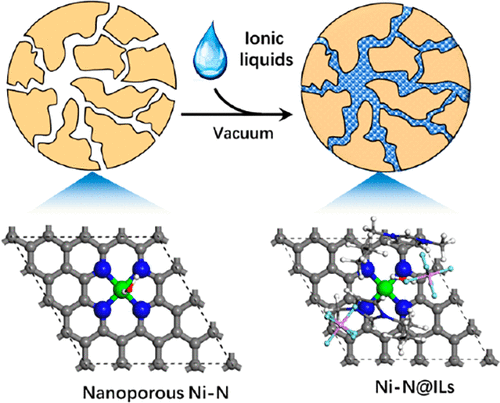
The development of strategies to enhance the electrocatalytic properties of single-atom catalysts is highly desirable, yet challenging. Here we show a versatile nanoconfined ionic liquids (ILs) design to tune the interactions between CO2 and single-Ni-site, meanwhile create a solid/liquid interface with high CO2 concentration for efficient CO2 electrocatalysis. The Ni–N catalyst confined with ILs can be directly used with aqueous electrolytes to mitigate the mass transport and conductivity issues typically associated with viscous bulk ILs electrolytes. Density functional theory studies reveal that the d-band center of Ni atoms is positively shifted to the Fermi level in the presence of IL, and consequently the energy barrier of the rate-limiting step of CO2 reduction, CO2(g) → COOH*, drops significantly from 1.49 to 0.80 eV. The nanoconfined ILs intensify CO2 conversion by showing a high CO Faradaic efficiency of above 98%, enhanced current densities up to 66.1 mA cm–2, and robust stability over 50 h.
中文翻译:

在单镍站点上限制离子液体的存在会促进水性电解质中CO 2的电还原
迫切需要开发增强单原子催化剂的电催化性能的策略。在这里,我们展示了一种通用的纳米约束离子液体(IL)设计,可调节CO 2与单个Ni部位之间的相互作用,同时创建具有高CO 2浓度的固/液界面,以进行有效的CO 2电催化。含ILs的Ni-N催化剂可直接与水性电解质一起使用,以减轻通常与粘性大体积ILs电解质相关的传质和电导率问题。密度泛函理论研究表明,在存在IL的情况下,Ni原子的d谱带中心正向费米能级移动,因此,CO限速步骤的能垒减少2,CO 2(g)→COOH *从1.49大幅下降至0.80 eV。纳米限制的离子液体通过显示98%以上的高CO法拉第效率,高达66.1 mA cm –2的增强的电流密度以及在50小时内的稳定稳定性,从而增强了CO 2转化。
更新日期:2020-11-21
中文翻译:

在单镍站点上限制离子液体的存在会促进水性电解质中CO 2的电还原
迫切需要开发增强单原子催化剂的电催化性能的策略。在这里,我们展示了一种通用的纳米约束离子液体(IL)设计,可调节CO 2与单个Ni部位之间的相互作用,同时创建具有高CO 2浓度的固/液界面,以进行有效的CO 2电催化。含ILs的Ni-N催化剂可直接与水性电解质一起使用,以减轻通常与粘性大体积ILs电解质相关的传质和电导率问题。密度泛函理论研究表明,在存在IL的情况下,Ni原子的d谱带中心正向费米能级移动,因此,CO限速步骤的能垒减少2,CO 2(g)→COOH *从1.49大幅下降至0.80 eV。纳米限制的离子液体通过显示98%以上的高CO法拉第效率,高达66.1 mA cm –2的增强的电流密度以及在50小时内的稳定稳定性,从而增强了CO 2转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号