当前位置:
X-MOL 学术
›
Prog. Nucl. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of polyaniline nanocomposites in the trapping of thorium ions from aqueous solutions:Adsorption equilibrium, kinetics and thermodynamics
Progress in Nuclear Energy ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.pnucene.2020.103537 Sara Abdi , Masoud Nasiri , Mohammad Hasan Khani
Progress in Nuclear Energy ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.pnucene.2020.103537 Sara Abdi , Masoud Nasiri , Mohammad Hasan Khani
|
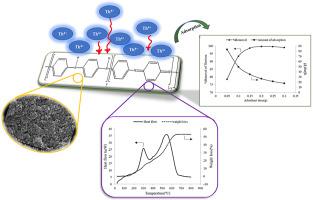
Abstract In this study, the adsorption behavior of Th(IV) ions was investigated using polyaniline (PAni) nanocomposites. Nanocomposites were synthesized with two types of dopants (H3PO4 and H2SO4) and two oxidants ((NH4)2S2O8 and KIO3) in the presence of two different surfactants (sodium dodecylbenzene sulfonate (SDBS) and hydroxypropyl cellulose (HPC)). The structure of the synthesized polymers was examined using FT-IR and SEM analyses. Adsorption of Th(IV) ions onto the synthesized polymers with a supreme removal (99.98%) was obtained by the H3PO4-doped polymers, especially for the sample with KIO3 oxidant and SDBS surfactant (PAni-2-1-S). BET, TGA and DSC analyses were also employed for the adsorbent characterization. The effect of main parameters was also investigated for the removal of Th(IV) ions from aqueous solutions by PAni-2-1-S. The pseudo-second-order kinetic and Langmuir isotherm models were in full agreement with the kinetic and isotherm experimental data, respectively and the highest adsorption capacity of the PAni-2-1-S was observed to be 68.49 mg/g. Thermodynamic data demonstrated the spontaneous and endothermic behavior of this adsorption process.
中文翻译:

聚苯胺纳米复合材料在水溶液中钍离子捕获中的应用:吸附平衡、动力学和热力学
摘要 在这项研究中,使用聚苯胺 (PAni) 纳米复合材料研究了 Th(IV) 离子的吸附行为。在两种不同的表面活性剂(十二烷基苯磺酸钠 (SDBS) 和羟丙基纤维素 (HPC))存在下,用两种掺杂剂(H3PO4 和 H2SO4)和两种氧化剂((NH4)2S2O8 和 KIO3)合成纳米复合材料。使用 FT-IR 和 SEM 分析检查合成聚合物的结构。通过 H3PO4 掺杂的聚合物,特别是对于含有 KIO3 氧化剂和 SDBS 表面活性剂 (PAni-2-1-S) 的样品,Th(IV) 离子以最高去除率(99.98%)吸附到合成聚合物上。BET、TGA 和 DSC 分析也用于吸附剂表征。还研究了主要参数对 PAni-2-1-S 从水溶液中去除 Th(IV) 离子的影响。拟二级动力学和朗缪尔等温线模型分别与动力学和等温线实验数据完全一致,观察到 PAni-2-1-S 的最高吸附容量为 68.49 mg/g。热力学数据证明了这种吸附过程的自发和吸热行为。
更新日期:2020-12-01
中文翻译:

聚苯胺纳米复合材料在水溶液中钍离子捕获中的应用:吸附平衡、动力学和热力学
摘要 在这项研究中,使用聚苯胺 (PAni) 纳米复合材料研究了 Th(IV) 离子的吸附行为。在两种不同的表面活性剂(十二烷基苯磺酸钠 (SDBS) 和羟丙基纤维素 (HPC))存在下,用两种掺杂剂(H3PO4 和 H2SO4)和两种氧化剂((NH4)2S2O8 和 KIO3)合成纳米复合材料。使用 FT-IR 和 SEM 分析检查合成聚合物的结构。通过 H3PO4 掺杂的聚合物,特别是对于含有 KIO3 氧化剂和 SDBS 表面活性剂 (PAni-2-1-S) 的样品,Th(IV) 离子以最高去除率(99.98%)吸附到合成聚合物上。BET、TGA 和 DSC 分析也用于吸附剂表征。还研究了主要参数对 PAni-2-1-S 从水溶液中去除 Th(IV) 离子的影响。拟二级动力学和朗缪尔等温线模型分别与动力学和等温线实验数据完全一致,观察到 PAni-2-1-S 的最高吸附容量为 68.49 mg/g。热力学数据证明了这种吸附过程的自发和吸热行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号