当前位置:
X-MOL 学术
›
Mater. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vanadium dioxide nanoparticles as a promising sorbent for controlled removal of waterborne fluoroquinolone ciprofloxacin
Materials Chemistry and Physics ( IF 4.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.matchemphys.2020.123993 Nahid Tavakkoli Nezhad , Mahmoud Shams , Aliakbar Dehghan , Mohammad Aziznezhad , Elaheh K. Goharshadi , Fatemeh Mohammadhosseini , Lee D. Wilson
Materials Chemistry and Physics ( IF 4.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.matchemphys.2020.123993 Nahid Tavakkoli Nezhad , Mahmoud Shams , Aliakbar Dehghan , Mohammad Aziznezhad , Elaheh K. Goharshadi , Fatemeh Mohammadhosseini , Lee D. Wilson
|
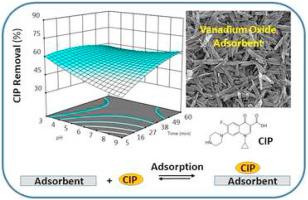
Abstract As recognized by international agencies, the uncontrolled release of antibiotics into global water resources have raised serious water security concerns. Herein, the uptake of a known persistent fluoroquinolone agent (ciprofloxacin; CIP) was studied by characterization of the adsorption properties of a novel metal oxide in aqueous media. Belt-like nanostructured vanadium dioxide (VO2) with specific surface area of 27.6 m2/g was prepared via a hydrothermal process that revealed exceptional chemical and physical properties related to its use as an adsorbent for CIP. The efficiency of the adsorption process was established using the Box–Behnken design (BBD) to determine optimal operational parameters: pH (4-10), contact time (5-60 min), CIP level (25-100 mg/L), and VO2 dosage (0.1-0.5 g/L). The BBD model enabled determination of the optimum CIP removal efficiency by considering different variables such as the adsorbent dosage, mixing time, pH, and CIP concentration. According to BBD model, the optimized parameters for the highest uptake of CIP (theoretically 100%) are pH 7, mixing time ∼ 58.3 min, CIP concentration (27.6 mg/L), and VO2 dosage (0.49 g/L). The maximum adsorption capacity of CIP was calculated as 102.2 mg/g by the Langmuir isotherm model. The pseudo-second order kinetic model revealed that chemisorption was a rate-limiting step for the kinetic uptake process, in agreement with an ion-exchange mechanism based on argument of ionic charge on the adsorbate and adsorbent as optimized conditions. The range of separation factor (0.01 to 0.09) values revealed that the process of CIP removal by VO2 is favorable. Regeneration of the VO2 adsorbent was demonstrated by use of a diluted HCl solution, where a slight decrease (ca. 10%) in adsorption performance was evidenced over several cycles of adsorption-desorption.
中文翻译:

二氧化钒纳米粒子作为一种有前途的吸附剂,用于控制去除水性氟喹诺酮环丙沙星
摘要 正如国际机构所承认的,抗生素不受控制地释放到全球水资源中已经引起了严重的水安全问题。在此,通过表征新型金属氧化物在水性介质中的吸附特性,研究了已知的持久性氟喹诺酮类药物(环丙沙星;CIP)的吸收。比表面积为 27.6 m2/g 的带状纳米二氧化钒 (VO2) 是通过水热工艺制备的,该工艺显示出与用作 CIP 吸附剂相关的特殊化学和物理特性。使用 Box-Behnken 设计 (BBD) 确定吸附过程的效率,以确定最佳操作参数:pH (4-10)、接触时间 (5-60 分钟)、CIP 水平 (25-100 mg/L)、和 VO2 剂量 (0.1-0.5 g/L)。BBD 模型可以通过考虑不同的变量(例如吸附剂剂量、混合时间、pH 值和 CIP 浓度)来确定最佳 CIP 去除效率。根据 BBD 模型,CIP 最高吸收率(理论上 100%)的优化参数是 pH 7、混合时间 ~ 58.3 分钟、CIP 浓度(27.6 mg/L)和 VO2 剂量(0.49 g/L)。通过朗缪尔等温线模型计算出 CIP 的最大吸附容量为 102.2 mg/g。伪二级动力学模型表明,化学吸附是动力学吸收过程的限速步骤,这与基于吸附质和吸附剂上的离子电荷作为优化条件的论点的离子交换机制一致。分离因子 (0.01 到 0.09) 值的范围表明通过 VO2 去除 CIP 的过程是有利的。
更新日期:2021-02-01
中文翻译:

二氧化钒纳米粒子作为一种有前途的吸附剂,用于控制去除水性氟喹诺酮环丙沙星
摘要 正如国际机构所承认的,抗生素不受控制地释放到全球水资源中已经引起了严重的水安全问题。在此,通过表征新型金属氧化物在水性介质中的吸附特性,研究了已知的持久性氟喹诺酮类药物(环丙沙星;CIP)的吸收。比表面积为 27.6 m2/g 的带状纳米二氧化钒 (VO2) 是通过水热工艺制备的,该工艺显示出与用作 CIP 吸附剂相关的特殊化学和物理特性。使用 Box-Behnken 设计 (BBD) 确定吸附过程的效率,以确定最佳操作参数:pH (4-10)、接触时间 (5-60 分钟)、CIP 水平 (25-100 mg/L)、和 VO2 剂量 (0.1-0.5 g/L)。BBD 模型可以通过考虑不同的变量(例如吸附剂剂量、混合时间、pH 值和 CIP 浓度)来确定最佳 CIP 去除效率。根据 BBD 模型,CIP 最高吸收率(理论上 100%)的优化参数是 pH 7、混合时间 ~ 58.3 分钟、CIP 浓度(27.6 mg/L)和 VO2 剂量(0.49 g/L)。通过朗缪尔等温线模型计算出 CIP 的最大吸附容量为 102.2 mg/g。伪二级动力学模型表明,化学吸附是动力学吸收过程的限速步骤,这与基于吸附质和吸附剂上的离子电荷作为优化条件的论点的离子交换机制一致。分离因子 (0.01 到 0.09) 值的范围表明通过 VO2 去除 CIP 的过程是有利的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号