当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tabernaesines A–I, Cytotoxic Aspidosperma–Aspidosperma-Type Bisindole Alkaloids from Tabernaemontana pachysiphon
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.jnatprod.9b00768 Wen-Fang Yi 1 , Xiao Ding 1 , Yuan-Zhi Chen 1 , Tiwalade A Adelakun 1 , Yu Zhang 1 , Xiao-Jiang Hao 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.jnatprod.9b00768 Wen-Fang Yi 1 , Xiao Ding 1 , Yuan-Zhi Chen 1 , Tiwalade A Adelakun 1 , Yu Zhang 1 , Xiao-Jiang Hao 1
Affiliation
|
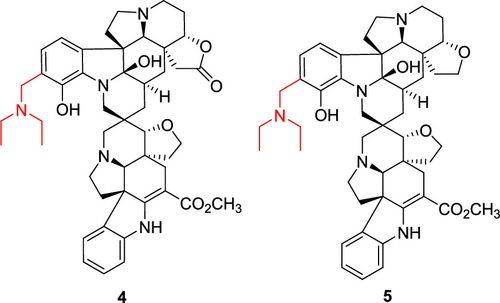
Twenty-one aspidosperma–aspidosperma alkaloids, including the new tabernaesines A–J (1–9), were obtained from Tabernaemontana pachysiphon. The structures and absolute configurations were elucidated using HRMS and NMR experiments. Compounds 1–9 possessed a rare spiro heterocycle moiety between the monomeric units, while compounds 4 and 5 were characterized by an indole ring fused with an (N,N-diethyl)methyl amino group. Compounds 1, 5–7, 15, and 16 exhibited moderate cytotoxic potency against various human cancer cell lines at IC50 2.5–9.8 μM.
中文翻译:

Tabernaesines A–I,来自 Tabernaemontana pachysiphon 的细胞毒性 Aspidosperma-Aspidosperma-Type Bisindole 生物碱
二十一白坚木属-白坚木属生物碱,包括新的tabernaesines甲-J(1 - 9),为得自狗牙花属pachysiphon。使用 HRMS 和 NMR 实验阐明了结构和绝对构型。化合物1 - 9所具有的单体单元之间的稀有螺杂环部分,而化合物4和5是由与(稠合的吲哚环,其特征在于Ñ,ñ -二乙基)甲基氨基。化合物1,5 - 7,15,和16在 IC 50 2.5–9.8 μM 时对各种人类癌细胞系表现出中等的细胞毒性效力。
更新日期:2020-11-25
中文翻译:

Tabernaesines A–I,来自 Tabernaemontana pachysiphon 的细胞毒性 Aspidosperma-Aspidosperma-Type Bisindole 生物碱
二十一白坚木属-白坚木属生物碱,包括新的tabernaesines甲-J(1 - 9),为得自狗牙花属pachysiphon。使用 HRMS 和 NMR 实验阐明了结构和绝对构型。化合物1 - 9所具有的单体单元之间的稀有螺杂环部分,而化合物4和5是由与(稠合的吲哚环,其特征在于Ñ,ñ -二乙基)甲基氨基。化合物1,5 - 7,15,和16在 IC 50 2.5–9.8 μM 时对各种人类癌细胞系表现出中等的细胞毒性效力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号