当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of Cyanide with Hg0-Contaminated Gold Mining Tailings Produces Soluble Mercuric Cyanide Complexes
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.chemrestox.0c00211 Caryn S Seney 1 , Christy C Bridges 2 , Sumeja Aljic 1 , Matthew E Moore 1 , Sarah E Orr 2 , Mary C Barnes 2 , Lucy Joshee 2 , Olga N Uchakina 2 , Brian J Bellott 3 , Robert J McKallip 2 , Kevin Drace 4 , Marcello M Veiga 5 , Adam M Kiefer 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.chemrestox.0c00211 Caryn S Seney 1 , Christy C Bridges 2 , Sumeja Aljic 1 , Matthew E Moore 1 , Sarah E Orr 2 , Mary C Barnes 2 , Lucy Joshee 2 , Olga N Uchakina 2 , Brian J Bellott 3 , Robert J McKallip 2 , Kevin Drace 4 , Marcello M Veiga 5 , Adam M Kiefer 1
Affiliation
|
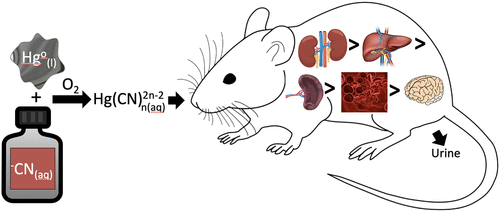
Elemental mercury (Hg0) contamination in artisanal and small-scale gold mining (ASGM) communities is widespread, and Hg0-contaminated tailings are often reprocessed with cyanide (−CN) to extract residual gold remaining after amalgamation. Hg0 reacts with –CN under aerobic conditions to produce Hg(CN)42– and other Hg(CN)nn–2 complexes. The production of solvated Hg(CN)nn–2 complexes increases upon agitation in the presence of synthetic and authentic Hg0-contaminated tailings that aid in dispersing the Hg0, increasing its reactive surface area. Adult rats were exposed to various concentrations of Hg(CN)2, and accumulation in organs and tissues was quantified using direct mercury analysis. The primary site of Hg(CN)2 accumulation was the kidney, although accumulation was also detected in the liver, spleen, and blood. Little accumulation was observed in the brain, suggesting that Hg(CN)2 complexes do not cross the blood–brain barrier. Renal tissue was particularly sensitive to the effects of Hg(CN)2, with pathological changes observed at low concentrations. Hg(CN)2 complexes are handled by mammalian systems in a manner similar to other inorganic species of Hg, yet appear to be more toxic to organ systems. The findings from this study are the first to show that Hg(CN)2 complexes are highly stable complexes that can lead to cellular injury and death in mammalian organ systems.
中文翻译:

氰化物与 Hg0 污染的金矿尾矿反应生成可溶性氰化汞配合物
手工和小规模采金业 (ASGM) 社区中的元素汞 (Hg 0 ) 污染很普遍,并且经常用氰化物 ( - CN) 对受Hg 0污染的尾矿进行再加工,以提取汞齐化后剩余的金。Hg 0在有氧条件下与– CN反应生成 Hg(CN) 4 2–和其他 Hg(CN) n n –2复合物。溶剂化 Hg(CN) n n –2复合物的产生在存在合成和真实 Hg 0污染的尾矿时搅拌增加,有助于分散 Hg 0,增加其反应表面积。成年大鼠暴露于不同浓度的 Hg(CN) 2,并使用直接汞分析量化器官和组织中的积累。Hg(CN) 2积累的主要部位是肾脏,尽管在肝脏、脾脏和血液中也检测到了积累。在大脑中观察到很少的积累,表明 Hg(CN) 2复合物不会穿过血脑屏障。肾组织对 Hg(CN) 2的影响特别敏感,在低浓度下观察到病理变化。汞(CN) 2复合物由哺乳动物系统以类似于其他无机汞物种的方式处理,但似乎对器官系统更具毒性。这项研究的结果首次表明 Hg(CN) 2复合物是高度稳定的复合物,可导致哺乳动物器官系统的细胞损伤和死亡。
更新日期:2020-11-16
中文翻译:

氰化物与 Hg0 污染的金矿尾矿反应生成可溶性氰化汞配合物
手工和小规模采金业 (ASGM) 社区中的元素汞 (Hg 0 ) 污染很普遍,并且经常用氰化物 ( - CN) 对受Hg 0污染的尾矿进行再加工,以提取汞齐化后剩余的金。Hg 0在有氧条件下与– CN反应生成 Hg(CN) 4 2–和其他 Hg(CN) n n –2复合物。溶剂化 Hg(CN) n n –2复合物的产生在存在合成和真实 Hg 0污染的尾矿时搅拌增加,有助于分散 Hg 0,增加其反应表面积。成年大鼠暴露于不同浓度的 Hg(CN) 2,并使用直接汞分析量化器官和组织中的积累。Hg(CN) 2积累的主要部位是肾脏,尽管在肝脏、脾脏和血液中也检测到了积累。在大脑中观察到很少的积累,表明 Hg(CN) 2复合物不会穿过血脑屏障。肾组织对 Hg(CN) 2的影响特别敏感,在低浓度下观察到病理变化。汞(CN) 2复合物由哺乳动物系统以类似于其他无机汞物种的方式处理,但似乎对器官系统更具毒性。这项研究的结果首次表明 Hg(CN) 2复合物是高度稳定的复合物,可导致哺乳动物器官系统的细胞损伤和死亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号