当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Tryptophan Residues in the Toxicity and Photosensitized Inactivation of Escherichia coli α-Hemolysin
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.biochem.0c00660 Lara O. Reid 1 , Andrés H. Thomas 1 , Vanesa Herlax 2 , M. Laura Dántola 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.biochem.0c00660 Lara O. Reid 1 , Andrés H. Thomas 1 , Vanesa Herlax 2 , M. Laura Dántola 1
Affiliation
|
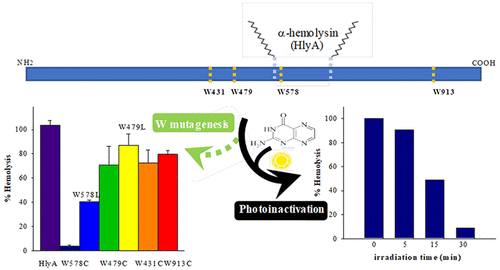
α-Hemolysin (HlyA) is an extracellular protein toxin secreted by uropathogenic strains of Escherichia coli that inserts into membranes of eukaryotic cells. The main goal of this work was to investigate the involvement of tryptophan (W) residues in the hemolytic activity of HlyA. We investigated the hemolytic activity of six single-point mutant proteins, in which one of the four Ws was replaced by cysteine (C) or leucine (L). We also analyzed the photoinactivation of HlyA with pterin (Ptr), an endogenous photosensitizer, as a method of unspecific oxidation of W and tyrosine (Y) residues. HlyA photoinactivation was analyzed by ultraviolet–visible spectrophotometry, hemolytic activity measurement, fluorescence spectroscopy, and electrophoretic analysis. The results indicate that Ws are important in the hemolytic process. Specifically, the chemical structure of the amino acid at position 578 is important for the acylation of HlyA at residue K563. Furthermore, the exposure of HlyA to ultraviolet radiation, with energy similar to that experienced under sun exposure, in the presence of Ptr induces the inactivation of the toxin, causing chemical changes in, at least, W and Y, the rate of damage to W residues being faster than that observed for Y residues. This work not only deepens our understanding of the structure–function relationship of the toxin but also introduces the possibility of using photoinactivation of HlyA for potential applications such as obtaining innocuous molecules for vaccine production and the elimination of the toxin from contaminated surfaces and drinking water.
中文翻译:

色氨酸残基在大肠杆菌α-溶血素的毒性和光敏灭活中的作用
α-溶血素(HlyA)是由大肠杆菌的泌尿道致病菌菌株分泌的细胞外蛋白毒素插入真核细胞膜。这项工作的主要目的是调查色氨酸(W)残留物参与HlyA的溶血活性。我们研究了六个单点突变蛋白的溶血活性,其中四个Ws之一被半胱氨酸(C)或亮氨酸(L)取代。我们还分析了内源性光敏剂蝶呤(Ptr)对HlyA的光灭活,作为W和酪氨酸(Y)残基的非特异性氧化方法。通过紫外线可见分光光度法,溶血活性测量,荧光光谱和电泳分析对HlyA的光灭活进行了分析。结果表明,Ws在溶血过程中很重要。具体地,在位置578的氨基酸的化学结构对于残基K563上的HlyA的酰化是重要的。此外,在Ptr的存在下,HlyA暴露于紫外线下,能量类似于在阳光下所经历的能量,会导致毒素失活,从而导致至少W和Y发生化学变化,从而破坏W残基比观察到的Y残基快。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。W和Y,对W残基的破坏速度快于对Y残基的破坏速度。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。W和Y,对W残基的破坏速度快于对Y残基的破坏速度。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。
更新日期:2020-11-12
中文翻译:

色氨酸残基在大肠杆菌α-溶血素的毒性和光敏灭活中的作用
α-溶血素(HlyA)是由大肠杆菌的泌尿道致病菌菌株分泌的细胞外蛋白毒素插入真核细胞膜。这项工作的主要目的是调查色氨酸(W)残留物参与HlyA的溶血活性。我们研究了六个单点突变蛋白的溶血活性,其中四个Ws之一被半胱氨酸(C)或亮氨酸(L)取代。我们还分析了内源性光敏剂蝶呤(Ptr)对HlyA的光灭活,作为W和酪氨酸(Y)残基的非特异性氧化方法。通过紫外线可见分光光度法,溶血活性测量,荧光光谱和电泳分析对HlyA的光灭活进行了分析。结果表明,Ws在溶血过程中很重要。具体地,在位置578的氨基酸的化学结构对于残基K563上的HlyA的酰化是重要的。此外,在Ptr的存在下,HlyA暴露于紫外线下,能量类似于在阳光下所经历的能量,会导致毒素失活,从而导致至少W和Y发生化学变化,从而破坏W残基比观察到的Y残基快。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。W和Y,对W残基的破坏速度快于对Y残基的破坏速度。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。W和Y,对W残基的破坏速度快于对Y残基的破坏速度。这项工作不仅加深了我们对毒素的结构-功能关系的理解,而且还引入了将HlyA光灭活用于潜在应用的可能性,例如获得无毒分子用于疫苗生产以及从被污染的表面和饮用水中消除毒素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号