当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Tiered Approach for Characterization to Ensure Quality, Reproducibility, and Long-Term Stability of Critical Reagents in Regulated Bioanalysis to Support PK/ADA/NAb Assays for Biologics and Vaccines Programs
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-10-28 , DOI: 10.1021/acsptsci.0c00135 Kun Yang 1 , Ying Zhang 2 , Robert Chou 2 , Lai Yeung 1 , Simon Letarte 3 , Rong-Sheng Yang 3 , Xuanwen Li 3 , Maribel Beaumont 4 , Rico Gunawan 2 , Douglas Richardson 3 , Shara Dellatore 1 , Eric Woolf 5 , Yang Xu 5
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-10-28 , DOI: 10.1021/acsptsci.0c00135 Kun Yang 1 , Ying Zhang 2 , Robert Chou 2 , Lai Yeung 1 , Simon Letarte 3 , Rong-Sheng Yang 3 , Xuanwen Li 3 , Maribel Beaumont 4 , Rico Gunawan 2 , Douglas Richardson 3 , Shara Dellatore 1 , Eric Woolf 5 , Yang Xu 5
Affiliation
|
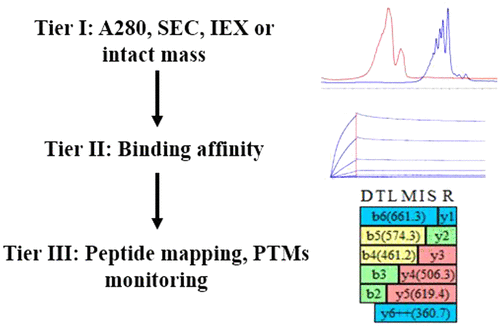
The robustness of good laboratory practice and clinical data is reliant upon a clear understanding of the bioanalytical assays. One of the most important components of ligand-binding based assays is critical reagents used to directly or indirectly measure biologic markers or signals. High quality, reproducible, sustainable critical reagents through the development lifecycle could avoid unnecessary rework, multiple validations, cross-validations, and ensure consistency of the data. Numerous analytical methods (UPLC-size exclusion chromatography, cation exchange chromatography, biacore/octet, and high-resolution mass spectrometry) have been evaluated by using current critical reagents. A comprehensive analytical toolbox of biochemical and biophysical methods has been employed to evaluate the quality of critical reagents and explore potential issues if there are any. Moving forward, this “tiered approach” of critical reagents characterization will be used not only to establish critical quality attributes for new reagents but also to evaluate stability in support of reagents recertification.
中文翻译:

一种分层表征方法,可确保受监管生物分析中关键试剂的质量、重现性和长期稳定性,以支持生物制品和疫苗项目的 PK/ADA/NAb 检测
良好的实验室实践和临床数据的稳健性依赖于对生物分析测定的清晰理解。基于配体结合的检测最重要的组成部分之一是用于直接或间接测量生物标志物或信号的关键试剂。在整个开发生命周期中使用高质量、可重复、可持续的关键试剂可以避免不必要的返工、多次验证、交叉验证,并确保数据的一致性。许多分析方法(UPLC 尺寸排阻色谱、阳离子交换色谱、biacore/octet 和高分辨率质谱)已通过使用当前的关键试剂进行了评估。已采用生化和生物物理方法的综合分析工具箱来评估关键试剂的质量并探索潜在问题(如果有的话)。展望未来,这种关键试剂表征的“分层方法”不仅将用于建立新试剂的关键质量属性,还将用于评估稳定性以支持试剂重新认证。
更新日期:2020-12-12
中文翻译:

一种分层表征方法,可确保受监管生物分析中关键试剂的质量、重现性和长期稳定性,以支持生物制品和疫苗项目的 PK/ADA/NAb 检测
良好的实验室实践和临床数据的稳健性依赖于对生物分析测定的清晰理解。基于配体结合的检测最重要的组成部分之一是用于直接或间接测量生物标志物或信号的关键试剂。在整个开发生命周期中使用高质量、可重复、可持续的关键试剂可以避免不必要的返工、多次验证、交叉验证,并确保数据的一致性。许多分析方法(UPLC 尺寸排阻色谱、阳离子交换色谱、biacore/octet 和高分辨率质谱)已通过使用当前的关键试剂进行了评估。已采用生化和生物物理方法的综合分析工具箱来评估关键试剂的质量并探索潜在问题(如果有的话)。展望未来,这种关键试剂表征的“分层方法”不仅将用于建立新试剂的关键质量属性,还将用于评估稳定性以支持试剂重新认证。











































 京公网安备 11010802027423号
京公网安备 11010802027423号