当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-State Competitive Destabilization of Caffeine Malonic Acid Cocrystal: Mechanistic and Kinetic Investigations
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.cgd.0c01246 Mhd. Bashir Alsirawan 1 , Xiaojun Lai 2 , Rafel Prohens 3 , Venu R. Vangala 1 , Sudhir K. Pagire 1 , Petroc Shelley 4 , Thomas J. Bannan 5 , David O. Topping 4, 5 , Anant Paradkar 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.cgd.0c01246 Mhd. Bashir Alsirawan 1 , Xiaojun Lai 2 , Rafel Prohens 3 , Venu R. Vangala 1 , Sudhir K. Pagire 1 , Petroc Shelley 4 , Thomas J. Bannan 5 , David O. Topping 4, 5 , Anant Paradkar 1
Affiliation
|
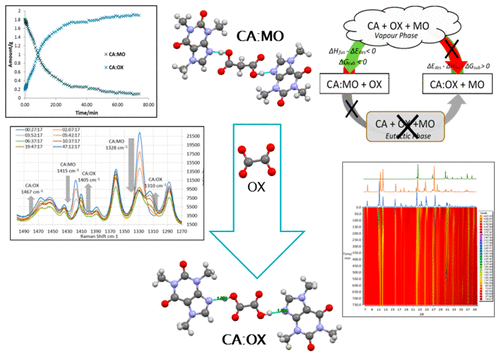
The main objective of this research is to investigate both the mechanism and the kinetics of solvent-free destabilization of the model caffeine/malonic acid cocrystal (CA/MO 2:1) in the presence of oxalic acid (OX) as a structural competitor to malonic acid (MO). Competitive destabilization of CA/MO and subsequent formation of caffeine/oxalic acid (CA/OX) take place at temperatures significantly below their melting points. The destabilization mechanism was found to be mediated by sublimation of both CA/MO and OX. During CA/MO destabilization, free CA could not be detected, and direct transformation to the CA/OX cocrystal was observed. The destabilization kinetics follow Prout–Tompkins nucleation and the crystal growth model with an activation energy of 133.91 kJ/mol, and subsequent CA/OX growth kinetics follow Ginstling–Brounshtien and three-dimensional diffusion models with an activation energy range of 130.45–132.57 kJ/mol.
中文翻译:

咖啡因丙二酸共晶体的固态竞争去稳定:机理和动力学研究。
这项研究的主要目的是研究在草酸(OX)作为结构竞争剂的情况下,模型咖啡因/丙二酸共晶体(CA / MO 2:1)的无溶剂去稳定机理和动力学。丙二酸(MO)。CA / MO的竞争性不稳定作用以及随后形成的咖啡因/草酸(CA / OX)的形成均在明显低于其熔点的温度下发生。发现去稳定机制由CA / MO和OX的升华介导。在CA / MO不稳定过程中,无法检测到游离CA,并且观察到直接转化为CA / OX共晶。不稳定动力学遵循Prout–Tompkins成核和晶体生长模型,其活化能为133.91 kJ / mol,
更新日期:2020-12-02
中文翻译:

咖啡因丙二酸共晶体的固态竞争去稳定:机理和动力学研究。
这项研究的主要目的是研究在草酸(OX)作为结构竞争剂的情况下,模型咖啡因/丙二酸共晶体(CA / MO 2:1)的无溶剂去稳定机理和动力学。丙二酸(MO)。CA / MO的竞争性不稳定作用以及随后形成的咖啡因/草酸(CA / OX)的形成均在明显低于其熔点的温度下发生。发现去稳定机制由CA / MO和OX的升华介导。在CA / MO不稳定过程中,无法检测到游离CA,并且观察到直接转化为CA / OX共晶。不稳定动力学遵循Prout–Tompkins成核和晶体生长模型,其活化能为133.91 kJ / mol,











































 京公网安备 11010802027423号
京公网安备 11010802027423号