当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoanalytical Identification of Siderite Dissolution-Coupled Pb Removal Mechanisms from Oxic and Anoxic Aqueous Solutions
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acsearthspacechem.0c00180 Lisa C. Füllenbach 1 , Jeffrey Paulo H. Perez 2 , Helen M. Freeman 2, 3 , Andrew N. Thomas 4 , Sathish Mayanna 2, 5 , Julia E. Parker 6 , Jörg Göttlicher 7 , Ralph Steininger 7 , Jörg Radnik 8 , Liane G. Benning 2, 9 , Eric H. Oelkers 1, 10
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acsearthspacechem.0c00180 Lisa C. Füllenbach 1 , Jeffrey Paulo H. Perez 2 , Helen M. Freeman 2, 3 , Andrew N. Thomas 4 , Sathish Mayanna 2, 5 , Julia E. Parker 6 , Jörg Göttlicher 7 , Ralph Steininger 7 , Jörg Radnik 8 , Liane G. Benning 2, 9 , Eric H. Oelkers 1, 10
Affiliation
|
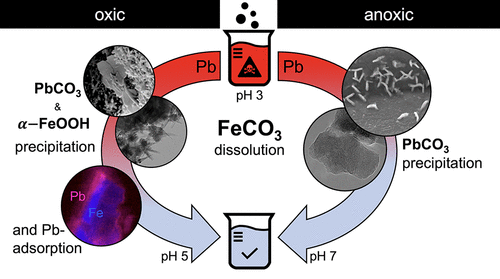
Lead(II) is a toxic pollutant often found in metal-contaminated soils and wastewaters. In acidic aqueous environments, Pb(II) is highly mobile. Chemical treatment strategies of such systems therefore often include neutralization agents and metal sorbents. Since metal solubility and the retention potential of sorbents depend on the redox state of the aqueous system, we tested the efficiency of the naturally occurring redox-sensitive ferrous iron carbonate mineral siderite to remove Pb(II) from acidic aqueous solutions in batch experiments under oxic and anoxic conditions over a total of 1008 h. Siderite dissolution led to an increase in reactive solution pH from 3 to 5.3 and 6.9, while 90 and 100% of the initial aqueous Pb(II) (0.48 × 10–3 mol kg–1) were removed from the oxic and anoxic systems, respectively. Scanning and transmission electron microscopy, combined with X-ray absorption and photoelectron spectroscopy, indicated that under oxic conditions, Pb(II) was consumed by cerussite precipitation and inner-sphere surface complexation to secondary goethite. Under anoxic conditions, Pb(II) was removed by the rapid precipitation of cerussite. This efficient siderite dissolution-coupled sequestration of Pb(II) into more stable solid phases demonstrates this potential method for contaminated water treatment regardless of the redox environment.
中文翻译:

含氧和无氧水溶液中菱铁矿溶解耦合的Pb去除机理的纳米分析鉴定
铅(II)是一种有毒污染物,经常在金属污染的土壤和废水中发现。在酸性水性环境中,Pb(II)具有很高的移动性。因此,此类系统的化学处理策略通常包括中和剂和金属吸附剂。由于金属的溶解度和吸附剂的保留潜力取决于水性体系的氧化还原状态,因此在有氧环境下的分批实验中,我们测试了天然存在的氧化还原敏感型碳酸亚铁碳酸铁矿矿物菱铁矿从酸性水溶液中去除Pb(II)的效率。和缺氧条件持续了1008小时。菱铁矿的溶解导致反应溶液的pH从3增加到5.3和6.9,而初始Pb(II)水溶液的90%和100%(0.48×10 –3 mol kg –1)分别从有氧和无氧系统中去除。扫描和透射电子显微镜,结合X射线吸收和光电子能谱,表明在有氧条件下,铜铈矿沉淀和内球面络合成次针铁矿会消耗Pb(II)。在缺氧条件下,铜铁矿的快速沉淀去除了Pb(II)。这种有效的菱铁矿溶解耦合的Pb(II)螯合成更稳定的固相,无论氧化还原环境如何,都证明了这种潜在的污水处理方法。
更新日期:2020-11-19
中文翻译:

含氧和无氧水溶液中菱铁矿溶解耦合的Pb去除机理的纳米分析鉴定
铅(II)是一种有毒污染物,经常在金属污染的土壤和废水中发现。在酸性水性环境中,Pb(II)具有很高的移动性。因此,此类系统的化学处理策略通常包括中和剂和金属吸附剂。由于金属的溶解度和吸附剂的保留潜力取决于水性体系的氧化还原状态,因此在有氧环境下的分批实验中,我们测试了天然存在的氧化还原敏感型碳酸亚铁碳酸铁矿矿物菱铁矿从酸性水溶液中去除Pb(II)的效率。和缺氧条件持续了1008小时。菱铁矿的溶解导致反应溶液的pH从3增加到5.3和6.9,而初始Pb(II)水溶液的90%和100%(0.48×10 –3 mol kg –1)分别从有氧和无氧系统中去除。扫描和透射电子显微镜,结合X射线吸收和光电子能谱,表明在有氧条件下,铜铈矿沉淀和内球面络合成次针铁矿会消耗Pb(II)。在缺氧条件下,铜铁矿的快速沉淀去除了Pb(II)。这种有效的菱铁矿溶解耦合的Pb(II)螯合成更稳定的固相,无论氧化还原环境如何,都证明了这种潜在的污水处理方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号