当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanisms of Hydrothermal Ketonic Decarboxylation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acsearthspacechem.0c00189 Kristin N. Johnson-Finn 1, 2 , Ian R. Gould 2 , Lynda B. Williams 3 , Hilairy E. Hartnett 2, 3 , Everett L. Shock 2, 3
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-10-27 , DOI: 10.1021/acsearthspacechem.0c00189 Kristin N. Johnson-Finn 1, 2 , Ian R. Gould 2 , Lynda B. Williams 3 , Hilairy E. Hartnett 2, 3 , Everett L. Shock 2, 3
Affiliation
|
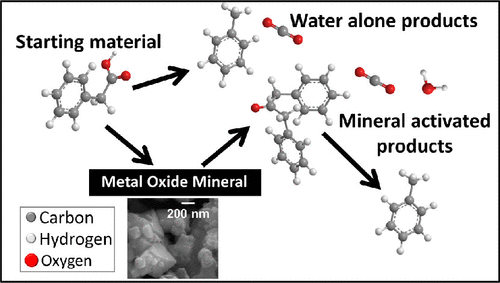
The formation of ketone products from carboxylic acids in the presence of minerals has not been considered in the interpretations of aqueous geochemistry, even though the formation of ketones is a well-known industrial process that occurs on mineral surfaces. This study demonstrates the formation of ketone products through ketonic decarboxylation from phenylacetic and hydrocinnamic acid in the presence of the mineral surfaces of magnetite (Fe3O4), hematite (Fe2O3), corundum (Al2O3), and spinel (MgAl2O4) at hydrothermal conditions (300 °C, 1000 bar). These minerals were chosen to deconvolve the mechanism of ketonic decarboxylation and explore the difference in abundance and rate of product formation on different kinds of oxide minerals. The presence of minerals increased the number and variety of reaction paths available to phenylacetic acid, compared to reactions without minerals. Magnetite and spinel favored the ketonic decarboxylation reaction more strongly than hematite and corundum, resulting in greater product yields. In the case of spinel, the presence of mineral both increases the formation of dibenzylketone and the decomposition of the same ketone into toluene.
中文翻译:

水热酮基脱羧的动力学和机理
尽管矿物质表面上发生的众所周知的工业过程,但酮的形成并未在含水地球化学的解释中考虑到在矿物存在下由羧酸形成酮产物。这项研究表明,在磁铁矿(Fe 3 O 4),赤铁矿(Fe 2 O 3),刚玉(Al 2 O 3)和尖晶石的矿物表面存在下,苯乙酸和氢肉桂酸通过酮基脱羧形成酮产物。(镁2 O 4)在水热条件下(300°C,1000 bar)。选择这些矿物是为了解开酮的脱羧机理,并探索不同种类的氧化物矿物在丰度和产物形成速率上的差异。与没有矿物质的反应相比,矿物质的存在增加了苯乙酸可用的反应路径的数量和种类。磁铁矿和尖晶石比赤铁矿和刚玉更有利于酮的脱羧反应,从而提高了产品收率。在尖晶石的情况下,矿物质的存在既增加了二苄基酮的形成,也增加了同一酮分解为甲苯的速度。
更新日期:2020-11-19
中文翻译:

水热酮基脱羧的动力学和机理
尽管矿物质表面上发生的众所周知的工业过程,但酮的形成并未在含水地球化学的解释中考虑到在矿物存在下由羧酸形成酮产物。这项研究表明,在磁铁矿(Fe 3 O 4),赤铁矿(Fe 2 O 3),刚玉(Al 2 O 3)和尖晶石的矿物表面存在下,苯乙酸和氢肉桂酸通过酮基脱羧形成酮产物。(镁2 O 4)在水热条件下(300°C,1000 bar)。选择这些矿物是为了解开酮的脱羧机理,并探索不同种类的氧化物矿物在丰度和产物形成速率上的差异。与没有矿物质的反应相比,矿物质的存在增加了苯乙酸可用的反应路径的数量和种类。磁铁矿和尖晶石比赤铁矿和刚玉更有利于酮的脱羧反应,从而提高了产品收率。在尖晶石的情况下,矿物质的存在既增加了二苄基酮的形成,也增加了同一酮分解为甲苯的速度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号