当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Completing the Redox‐Series of Silicon Trisdioxolene: ortho‐Quinone and Lewis Superacid Make a Powerful Redox Catalyst
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/chem.202004712 Rezisha Maskey 1 , Christoph Bendel 1 , Jonas Malzacher 1 , Lutz Greb 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/chem.202004712 Rezisha Maskey 1 , Christoph Bendel 1 , Jonas Malzacher 1 , Lutz Greb 1
Affiliation
|
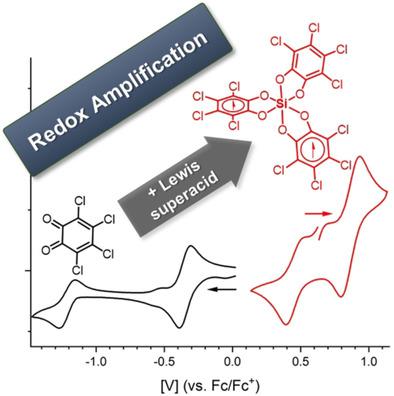
Quinones are mild oxidants, the redox potentials of which can be increased by supramolecular interactions. Whereas this goal has been achieved by hydrogen bonding or molecular encapsulation, a Lewis acid‐binding strategy for redox amplification of quinones is unexplored. Herein, the redox chemistry of silicon tris(perchloro)dioxolene 1 was studied, which is the formal adduct of ortho‐perchloroquinone QCl with the Lewis superacid bis(perchlorocatecholato)silane 2. By isolating the anionic monoradical 1.−, the redox‐series of a century‐old class of compounds was completed. Cyclic voltammetry measurements revealed that the redox potential in 1 was shifted by more than 1 V into the anodic direction compared to QCl, reaching that of “magic blue” or NO+. It allowed oxidation of challenging substrates such as aromatic hydrocarbons and could be applied as an efficient redox catalyst. Remarkably, this powerful reagent formed in situ by combining the two commercially available precursors SiI4 and QCl.
中文翻译:

完成硅三三恶酚的氧化还原系列:邻醌和路易斯超强酸成为强大的氧化还原催化剂
醌是温和的氧化剂,超分子相互作用可提高其氧化还原电位。尽管已经通过氢键或分子封装实现了这一目标,但尚未探索路易斯酸结合策略用于醌的氧化还原扩增。在本文中,研究了三(全氯)二氧杂环丁烯1硅的氧化还原化学,这是邻-全氯醌Q Cl与路易斯超酸双(全氯儿茶酚基)硅烷2的形式加合物。通过分离阴离子单自由基1 。−,已经完成了百年历史的化合物的氧化还原系列。循环伏安测量表明,在氧化还原电位1与Q Cl相比,它向阳极方向移动了1 V以上,达到了“魔术蓝”或NO +的水平。它可以氧化具有挑战性的底物,例如芳烃,并且可以用作有效的氧化还原催化剂。值得注意的是,这种强大的试剂是通过将两种市售前体SiI 4和Q Cl结合在一起而原位形成的。
更新日期:2020-12-23
中文翻译:

完成硅三三恶酚的氧化还原系列:邻醌和路易斯超强酸成为强大的氧化还原催化剂
醌是温和的氧化剂,超分子相互作用可提高其氧化还原电位。尽管已经通过氢键或分子封装实现了这一目标,但尚未探索路易斯酸结合策略用于醌的氧化还原扩增。在本文中,研究了三(全氯)二氧杂环丁烯1硅的氧化还原化学,这是邻-全氯醌Q Cl与路易斯超酸双(全氯儿茶酚基)硅烷2的形式加合物。通过分离阴离子单自由基1 。−,已经完成了百年历史的化合物的氧化还原系列。循环伏安测量表明,在氧化还原电位1与Q Cl相比,它向阳极方向移动了1 V以上,达到了“魔术蓝”或NO +的水平。它可以氧化具有挑战性的底物,例如芳烃,并且可以用作有效的氧化还原催化剂。值得注意的是,这种强大的试剂是通过将两种市售前体SiI 4和Q Cl结合在一起而原位形成的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号