当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Platinum ω-Alkenyl Compounds as Chemical Vapor Deposition Precursors. Mechanistic Studies of the Thermolysis of Pt[CH2CMe2CH2CH═CH2]2 in Solution and the Origin of Rapid Nucleation
Organometallics ( IF 2.5 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.organomet.0c00542 Sumeng Liu 1 , Zhejun Zhang 2 , John R. Abelson 2 , Gregory S. Girolami 1
Organometallics ( IF 2.5 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.organomet.0c00542 Sumeng Liu 1 , Zhejun Zhang 2 , John R. Abelson 2 , Gregory S. Girolami 1
Affiliation
|
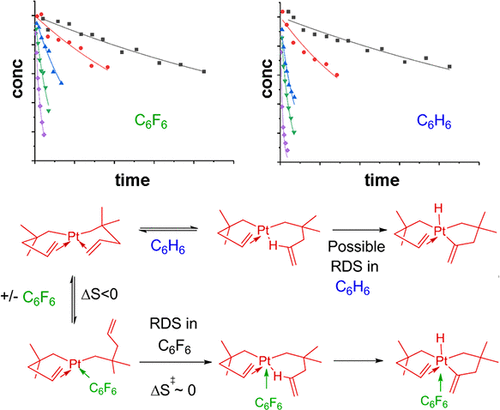
The compound cis-bis(η1,η2-2,2-dimethylpent-4-en-1-yl)platinum, Pt[CH2CMe2CH2CH═CH2]2 (3), is a recently discovered chemical vapor deposition (CVD) precursor for the deposition of highly smooth platinum thin films without nucleation delays on a variety of substrates. This paper describes detailed mechanistic studies of the pathway by which 3 reacts upon being heated in solution. In various solvents between 90 and 130 °C, 3 decomposes to generate ∼1 equiv of 4,4-dimethylpentenes by addition of a hydrogen atom to the pentenyl ligands in 3. The “extra” hydrogen atoms arise by dehydrogenation of other pentenyl ligands; some of these dehydrogenated ligands are released as methyl-substituted methylenecyclobutanes and cyclobutenes. A combination of isotope labeling and kinetic studies suggests that 3 decomposes by C–H activation of both allylic and olefinic C–H bonds to give transient platinum hydride intermediates, followed by reductive elimination steps to form the pentene products, but that the exact mechanism is solvent-dependent. In C6F6, solvent association occurs before C–H bond activation, and the rate-determining step for thermolysis is most likely the formation of a Pt σ complex. In hydrocarbon solvents, the solvent is little involved before C–H bond activation, and the rate-determining step is most likely the formation of a Pt σ complex only for γ-C–H and ε-C–H bond activation, but cleavage or formation of a C–H bond for δ-C–H bond activation. A comparison of the thermolysis reactions under CVD conditions and in solution suggests that the high smoothness of the CVD-grown films is due in part to rapid nucleation (which is a consequence of the availability of low-barrier C═C bond dissociation pathways) and in part to the formation of carbon-containing species that passivate the Pt surface.
中文翻译:

铂ω-烯基化合物作为化学气相沉积前体。铂的热分解[CH的机理的研究2 CME 2 CH 2 CH = CH 2 ] 2在溶液中,快速成核来源
化合物顺式-双(η 1,η 2 -2,2-二甲基戊-4-烯-1-基)铂,铂[CH 2 CME 2 CH 2 CH = CH 2 ] 2(3),是最近发现的化学气相沉积(CVD)前体,用于在各种基材上沉积高度光滑的铂薄膜,而没有成核延迟。本文介绍了3在溶液中加热时发生反应的途径的详细机理研究。在90至130°C之间的各种溶剂中,3通过分解成3个戊烯基配体中的氢原子,分解生成约1当量的4,4-二甲基戊烯。“额外的”氢原子是通过其他戊烯基配体的脱氢而产生的。这些脱氢配体中的一些以甲基取代的亚甲基环丁烷和环丁烯的形式释放。同位素标记和动力学研究的结合表明,3可以通过C–H烯丙基和烯烃C–H键的活化来分解,得到瞬态氢化铂中间体,然后通过还原消除步骤形成戊烯产物,但确切的机理是溶剂依赖性。在C 6 F 6,溶剂缔合发生在C–H键活化之前,而热解的速率决定步骤最有可能形成Ptσ络合物。在烃类溶剂中,在C–H键活化之前几乎不涉及溶剂,并且决定速率的步骤很可能仅在γ-C–H和ε-C–H键活化时形成Ptσ络合物,但会裂解或形成C–H键以激活δ–C–H键。在CVD条件下和溶液中进行热解反应的比较表明,CVD生长膜的高光滑度部分归因于快速成核作用(这是由于低阻隔C═C键解离途径的可利用性)和部分原因是钝化Pt表面的含碳物质的形成。
更新日期:2020-11-09
中文翻译:

铂ω-烯基化合物作为化学气相沉积前体。铂的热分解[CH的机理的研究2 CME 2 CH 2 CH = CH 2 ] 2在溶液中,快速成核来源
化合物顺式-双(η 1,η 2 -2,2-二甲基戊-4-烯-1-基)铂,铂[CH 2 CME 2 CH 2 CH = CH 2 ] 2(3),是最近发现的化学气相沉积(CVD)前体,用于在各种基材上沉积高度光滑的铂薄膜,而没有成核延迟。本文介绍了3在溶液中加热时发生反应的途径的详细机理研究。在90至130°C之间的各种溶剂中,3通过分解成3个戊烯基配体中的氢原子,分解生成约1当量的4,4-二甲基戊烯。“额外的”氢原子是通过其他戊烯基配体的脱氢而产生的。这些脱氢配体中的一些以甲基取代的亚甲基环丁烷和环丁烯的形式释放。同位素标记和动力学研究的结合表明,3可以通过C–H烯丙基和烯烃C–H键的活化来分解,得到瞬态氢化铂中间体,然后通过还原消除步骤形成戊烯产物,但确切的机理是溶剂依赖性。在C 6 F 6,溶剂缔合发生在C–H键活化之前,而热解的速率决定步骤最有可能形成Ptσ络合物。在烃类溶剂中,在C–H键活化之前几乎不涉及溶剂,并且决定速率的步骤很可能仅在γ-C–H和ε-C–H键活化时形成Ptσ络合物,但会裂解或形成C–H键以激活δ–C–H键。在CVD条件下和溶液中进行热解反应的比较表明,CVD生长膜的高光滑度部分归因于快速成核作用(这是由于低阻隔C═C键解离途径的可利用性)和部分原因是钝化Pt表面的含碳物质的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号