当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrile Oxidation at a Ruthenium Complex leading to Intermolecular Imido Group Transfer
Organometallics ( IF 2.5 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.organomet.0c00589 James E Bird 1 , Cole A Hammond 1 , Kjersti G Oberle 1 , Erin E Ramey 1 , Yutong Zou 1 , Ryan C Lash 1 , Christopher R Turlington 1
Organometallics ( IF 2.5 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.organomet.0c00589 James E Bird 1 , Cole A Hammond 1 , Kjersti G Oberle 1 , Erin E Ramey 1 , Yutong Zou 1 , Ryan C Lash 1 , Christopher R Turlington 1
Affiliation
|
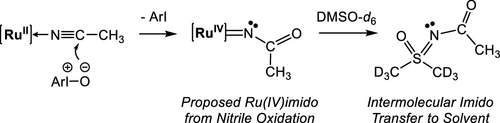
The oxidation of an acetonitrile ligand coordinated to ruthenium is explored in deuterated dimethyl sulfoxide by 1H NMR spectroscopy. When oxidized with an iodosoarene oxygen atom transfer (OAT) reagent, kinetic studies demonstrate that the nitrile ligand does not dissociate before reacting. Instead, OAT to the central nitrile carbon is implicated (nitrile oxidation) and is further supported by the product of the reaction, N-acyl-dimethylsulfoximine. The N-acyldimethylsulfoximine likely formed by an imido group transfer reaction from ruthenium to the NMR solvent, and the product was synthesized independently to verify its identity in the reaction. This reaction represents the first time that a nitrile oxidation reaction has resulted in intermolecular imido group transfer to a substrate, presumably through a reactive ruthenium(IV)imido intermediate. This suggests that nitrile oxidation is a plausible route into reactive metal–imido intermediates for amination and aziridination reactions.
中文翻译:

钌配合物中的腈氧化导致分子间亚氨基转移
通过1 H NMR 光谱研究了在氘代二甲基亚砜中与钌配位的乙腈配体的氧化。当用碘代芳烃氧原子转移 (OAT) 试剂氧化时,动力学研究表明腈配体在反应前不会解离。相反,OAT 与中心腈碳有关(腈氧化),并由反应产物N-酰基-二甲基亚砜亚胺进一步支持。该ñ-酰基二甲基亚砜亚胺可能是通过从钌到 NMR 溶剂的亚氨基转移反应形成的,并且该产物是独立合成的,以验证其在反应中的身份。该反应代表了腈氧化反应第一次导致分子间亚氨基转移到底物上,可能是通过反应性的钌 (IV) 亚氨基中间体。这表明腈氧化是用于胺化和氮丙啶化反应的活性金属-亚氨基中间体的可行途径。
更新日期:2020-11-09
中文翻译:

钌配合物中的腈氧化导致分子间亚氨基转移
通过1 H NMR 光谱研究了在氘代二甲基亚砜中与钌配位的乙腈配体的氧化。当用碘代芳烃氧原子转移 (OAT) 试剂氧化时,动力学研究表明腈配体在反应前不会解离。相反,OAT 与中心腈碳有关(腈氧化),并由反应产物N-酰基-二甲基亚砜亚胺进一步支持。该ñ-酰基二甲基亚砜亚胺可能是通过从钌到 NMR 溶剂的亚氨基转移反应形成的,并且该产物是独立合成的,以验证其在反应中的身份。该反应代表了腈氧化反应第一次导致分子间亚氨基转移到底物上,可能是通过反应性的钌 (IV) 亚氨基中间体。这表明腈氧化是用于胺化和氮丙啶化反应的活性金属-亚氨基中间体的可行途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号