当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The LnmK Bifunctional Acyltransferase/Decarboxylase Specifying (2R)-Methylmalonyl-CoA and Employing Substrate-Assisted Catalysis for Polyketide Biosynthesis
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-23 , DOI: 10.1021/acs.biochem.0c00749 Jeremy R Lohman 1 , Ben Shen
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-23 , DOI: 10.1021/acs.biochem.0c00749 Jeremy R Lohman 1 , Ben Shen
Affiliation
|
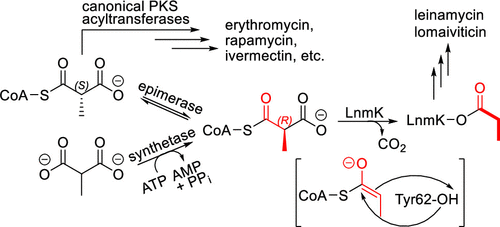
We previously showed that the bifunctional LnmK acyltransferase/decarboxylase (AT/DC) catalyzed the formation of a propionyl-S-acyl carrier protein (ACP) from methylmalonyl-CoA, but its substrate specificity to (2S)-, (2R)-, or (2RS)-methylmalonyl CoA was not known. We subsequently revealed that LnmK AT and DC activities share the same active site, employing a Tyr as the catalytic residue for AT, but failed to identify a general base within the vicinity of the active site for LnmK catalysis. We now show that (i) LnmK specifies (2R)-methylmalonyl-CoA and (ii) the AT and DC activities are coupled, featuring substrate-assisted catalysis via the enolate to account for the missing general base within the LnmK active site. LnmK and its homologues are the only bifunctional AT/DC enzymes known to date and are widespread. These findings, therefore, enrich PKS chemistry and enzymology. Since only the (2S)-methylmalonyl-CoA enantiomer has been established previously as a substrate for polyketide biosynthesis by PKSs, we now establish a role for both (2R)- and (2S)-methylmalonyl-CoA in polyketide biosynthesis, and (2R)-methylmalonyl-CoA should be considered as a substrate in future efforts for engineered production of polyketides by combinatorial biosynthesis or synthetic biology strategies in model hosts.
中文翻译:

LnmK 双功能酰基转移酶/脱羧酶指定 (2R)-甲基丙二酰辅酶 A 并使用底物辅助催化进行聚酮化合物生物合成
我们之前表明双功能 LnmK 酰基转移酶/脱羧酶 (AT/DC) 催化从甲基丙二酰辅酶 A形成丙酰基-S-酰基载体蛋白 (ACP),但其底物特异性为 (2 S )-, (2 R ) -, 或 (2 RS )-甲基丙二酰辅酶 A 未知。我们随后揭示了 LnmK AT 和 DC 活性共享相同的活性位点,使用 Tyr 作为 AT 的催化残基,但未能在 LnmK 催化活性位点附近确定一般碱基。我们现在证明 (i) LnmK 指定 (2 R)-甲基丙二酰辅酶 A 和 (ii) AT 和 DC 活性是耦合的,以通过烯醇化物的底物辅助催化为特征,以解决 LnmK 活性位点内缺失的一般碱基。LnmK 及其同源物是迄今为止已知的唯一双功能 AT/DC 酶,并且广泛使用。因此,这些发现丰富了 PKS 化学和酶学。由于之前仅将 (2 S )-甲基丙二酰-CoA 对映体确定为 PKS 聚酮化合物生物合成的底物,我们现在确定 (2 R )- 和 (2 S )-甲基丙二酰-CoA 在聚酮化合物生物合成中的作用,和 (2 R)-甲基丙二酰辅酶A应被视为未来通过组合生物合成或模型宿主中的合成生物学策略工程化生产聚酮化合物的底物。
更新日期:2020-11-03
中文翻译:

LnmK 双功能酰基转移酶/脱羧酶指定 (2R)-甲基丙二酰辅酶 A 并使用底物辅助催化进行聚酮化合物生物合成
我们之前表明双功能 LnmK 酰基转移酶/脱羧酶 (AT/DC) 催化从甲基丙二酰辅酶 A形成丙酰基-S-酰基载体蛋白 (ACP),但其底物特异性为 (2 S )-, (2 R ) -, 或 (2 RS )-甲基丙二酰辅酶 A 未知。我们随后揭示了 LnmK AT 和 DC 活性共享相同的活性位点,使用 Tyr 作为 AT 的催化残基,但未能在 LnmK 催化活性位点附近确定一般碱基。我们现在证明 (i) LnmK 指定 (2 R)-甲基丙二酰辅酶 A 和 (ii) AT 和 DC 活性是耦合的,以通过烯醇化物的底物辅助催化为特征,以解决 LnmK 活性位点内缺失的一般碱基。LnmK 及其同源物是迄今为止已知的唯一双功能 AT/DC 酶,并且广泛使用。因此,这些发现丰富了 PKS 化学和酶学。由于之前仅将 (2 S )-甲基丙二酰-CoA 对映体确定为 PKS 聚酮化合物生物合成的底物,我们现在确定 (2 R )- 和 (2 S )-甲基丙二酰-CoA 在聚酮化合物生物合成中的作用,和 (2 R)-甲基丙二酰辅酶A应被视为未来通过组合生物合成或模型宿主中的合成生物学策略工程化生产聚酮化合物的底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号