当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assembly of Plant Enzymes in E. coli for the Production of the Valuable (−)-Podophyllotoxin Precursor (−)-Pluviatolide
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-10-23 , DOI: 10.1021/acssynbio.0c00354 Davide Decembrino 1 , Esther Ricklefs 1 , Stefan Wohlgemuth 1 , Marco Girhard 1 , Katrin Schullehner 2 , Guido Jach 2 , Vlada B Urlacher 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-10-23 , DOI: 10.1021/acssynbio.0c00354 Davide Decembrino 1 , Esther Ricklefs 1 , Stefan Wohlgemuth 1 , Marco Girhard 1 , Katrin Schullehner 2 , Guido Jach 2 , Vlada B Urlacher 1
Affiliation
|
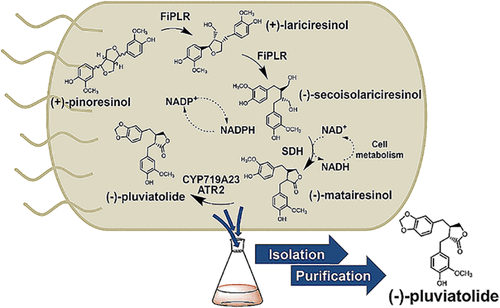
Lignans are plant secondary metabolites with a wide range of reported health-promoting bioactivities. Traditional routes toward these natural products involve, among others, the extraction from plant sources and chemical synthesis. However, the availability of the sources and the complex chemical structures of lignans often limit the feasibility of these approaches. In this work, we introduce a newly assembled biosynthetic route in E. coli for the efficient conversion of the common higher-lignan precursor (+)-pinoresinol to the noncommercially available (−)-pluviatolide via three intermediates. (−)-Pluviatolide is considered a crossroad compound in lignan biosynthesis, because the methylenedioxy bridge in its structure, resulting from the oxidation of (−)-matairesinol, channels the biosynthetic pathway toward the microtubule depolymerizer (−)-podophyllotoxin. This oxidation reaction is catalyzed with high regio- and enantioselectivity by a cytochrome P450 monooxygenase from Sinopodophyllum hexandrum (CYP719A23), which was expressed and optimized regarding redox partners in E. coli. Pinoresinol-lariciresinol reductase from Forsythia intermedia (FiPLR), secoisolariciresinol dehydrogenase from Podophyllum pleianthum (PpSDH), and CYP719A23 were coexpressed together with a suitable NADPH-dependent reductase to ensure P450 activity, allowing for four sequential biotransformations without intermediate isolation. By using an E. coli strain coexpressing the enzymes originating from four plants, (+)-pinoresinol was efficiently converted, allowing the isolation of enantiopure (−)-pluviatolide at a concentration of 137 mg/L (ee ≥99% with 76% isolated yield).
中文翻译:

在大肠杆菌中组装植物酶,用于生产有价值的 (-)-鬼臼毒素前体 (-)-Pluviatolide
木脂素是植物次生代谢产物,据报道具有广泛的促进健康的生物活性。这些天然产品的传统途径包括从植物来源中提取和化学合成。然而,来源的可用性和木脂素的复杂化学结构通常限制了这些方法的可行性。在这项工作中,我们在大肠杆菌中引入了一种新组装的生物合成路线,用于将常见的高级木脂素前体 (+)-松脂醇通过三个中间体。(-)-Pluviatolide 被认为是木脂素生物合成中的一种交叉化合物,因为其结构中的亚甲二氧基桥是由 (-)-matairesinol 氧化产生的,将生物合成途径引导至微管解聚剂 (-)-鬼臼毒素。该氧化反应由来自中华鬼臼属植物的细胞色素 P450 单加氧酶(CYP719A23)以高区域选择性和对映选择性催化,该酶在大肠杆菌中针对氧化还原伙伴进行表达和优化。来自中间连翘(FiPLR) 的松脂醇-落叶松树脂醇还原酶、来自鬼臼的开环异落叶松树脂醇脱氢酶(PpSDH) 和 CYP719A23 与合适的 NADPH 依赖性还原酶共表达以确保 P450 活性,允许进行四次连续生物转化而无需中间分离。通过使用共表达源自四种植物的酶的大肠杆菌菌株,(+)-松脂醇被有效转化,从而可以分离出浓度为 137 mg/L(ee ≥99% 和 76%分离产率)。
更新日期:2020-11-21
中文翻译:

在大肠杆菌中组装植物酶,用于生产有价值的 (-)-鬼臼毒素前体 (-)-Pluviatolide
木脂素是植物次生代谢产物,据报道具有广泛的促进健康的生物活性。这些天然产品的传统途径包括从植物来源中提取和化学合成。然而,来源的可用性和木脂素的复杂化学结构通常限制了这些方法的可行性。在这项工作中,我们在大肠杆菌中引入了一种新组装的生物合成路线,用于将常见的高级木脂素前体 (+)-松脂醇通过三个中间体。(-)-Pluviatolide 被认为是木脂素生物合成中的一种交叉化合物,因为其结构中的亚甲二氧基桥是由 (-)-matairesinol 氧化产生的,将生物合成途径引导至微管解聚剂 (-)-鬼臼毒素。该氧化反应由来自中华鬼臼属植物的细胞色素 P450 单加氧酶(CYP719A23)以高区域选择性和对映选择性催化,该酶在大肠杆菌中针对氧化还原伙伴进行表达和优化。来自中间连翘(FiPLR) 的松脂醇-落叶松树脂醇还原酶、来自鬼臼的开环异落叶松树脂醇脱氢酶(PpSDH) 和 CYP719A23 与合适的 NADPH 依赖性还原酶共表达以确保 P450 活性,允许进行四次连续生物转化而无需中间分离。通过使用共表达源自四种植物的酶的大肠杆菌菌株,(+)-松脂醇被有效转化,从而可以分离出浓度为 137 mg/L(ee ≥99% 和 76%分离产率)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号