当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introduction of cell-selectivity in bovine cathelicidin BMAP-28 by exchanging heptadic isoleucine with the adjacent proline at a non-heptadic position
Peptide Science ( IF 1.5 ) Pub Date : 2020-10-23 , DOI: 10.1002/pep2.24207 Sarfuddin Azmi 1, 2 , Neeraj Kumar Verma 1 , Jitendra Kumar Tripathi 1 , Saurabh Srivastava 1 , Devesh Pratap Verma 1 , Jimut Kanti Ghosh 1
Peptide Science ( IF 1.5 ) Pub Date : 2020-10-23 , DOI: 10.1002/pep2.24207 Sarfuddin Azmi 1, 2 , Neeraj Kumar Verma 1 , Jitendra Kumar Tripathi 1 , Saurabh Srivastava 1 , Devesh Pratap Verma 1 , Jimut Kanti Ghosh 1
Affiliation
|
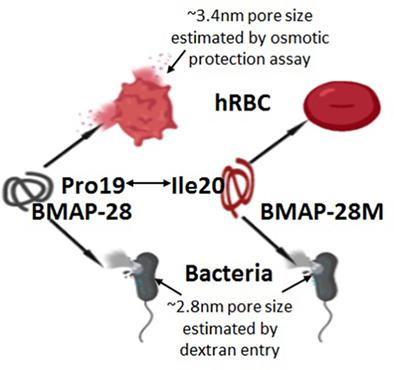
Bovine cathelicidin, BMAP-28 exhibits potent antimicrobial activity without any cell-selectivity. Considering the role of its C-terminus amino acid residues in its cytotoxic activities, we envisioned to rearrange the amino acids at its C-terminus without altering its composition for designing new cell-selective BMAP-28-analog. Thus an isoleucine residue at 20th position, also an “a” position of its previously identified isoleucine zipper sequence, was exchanged with a proline residue at 19th position. As a result, a remarkable reduction in the hemolytic activity of BMAP-28 toward human red blood cells (hRBCs) was observed without hampering its antibacterial activity in liquid media, and in presence of salts and serum. BMAP-28-analog, BMAP-28M depolarized S. aureus membrane and bacterial membrane-mimetic, PE/PG lipid vesicles similar to BMAP-28 but showed weaker efficacy to depolarize hRBC membrane and mammalian membrane-mimetic, PC/Chol vesicles. Differences in the localization of tryptophan residues of BMAP-28 and BMAP-28M were observed only in PC/Chol vesicles. BMAP-28 induced pores of ~3.4 nm diameter onto hRBCs whereas BMAP-28M showed minor perturbation onto these cells. BMAP-28 and BMAP-28M induced the entry of calcein (size, ~1 nm) and 4.4 kDa FITC-dextran (size, ~2.8 nm) into B. megaterium but not of higher sized fluorescent-dextrans indicating toroidal pore formation onto these bacterial membranes.
中文翻译:

通过在非七肽位置与邻近的脯氨酸交换七异异亮氨酸在牛cathelicidin BMAP-28中引入细胞选择性
牛cathelicidin BMAP-28表现出强大的抗菌活性,而没有任何细胞选择性。考虑到其C末端氨基酸残基在其细胞毒性活性中的作用,我们设想重新排列其C末端的氨基酸而不改变其组成,以设计新的细胞选择性BMAP-28-analog。因此,在第20位的异亮氨酸残基,也是其先前鉴定的异亮氨酸拉链序列的“ a”位,与在第19位的脯氨酸残基交换。结果,观察到BMAP-28对人红细胞(hRBCs)的溶血活性显着降低,而没有在液体介质中以及存在盐和血清的情况下抑制其抗菌活性。BMAP-28类似物,BMAP-28M去极化金黄色葡萄球菌膜和细菌膜模拟PE / PG脂质囊泡类似于BMAP-28,但显示出对hRBC膜和哺乳动物膜模拟PC / Chol囊泡去极化的功效较弱。仅在PC / Chol囊泡中观察到BMAP-28和BMAP-28M色氨酸残基的定位差异。BMAP-28在hRBC上诱导了直径约3.4 nm的孔,而BMAP-28M在这些细胞上显示出较小的扰动。BMAP-28和BMAP-28M诱导钙黄绿素(尺寸约1 nm)和4.4 kDa FITC-葡聚糖(尺寸约2.8 nm)进入巨大芽孢杆菌中,但未引入较大尺寸的荧光葡聚糖,表明在其上形成了环形孔细菌膜。
更新日期:2020-10-23
中文翻译:

通过在非七肽位置与邻近的脯氨酸交换七异异亮氨酸在牛cathelicidin BMAP-28中引入细胞选择性
牛cathelicidin BMAP-28表现出强大的抗菌活性,而没有任何细胞选择性。考虑到其C末端氨基酸残基在其细胞毒性活性中的作用,我们设想重新排列其C末端的氨基酸而不改变其组成,以设计新的细胞选择性BMAP-28-analog。因此,在第20位的异亮氨酸残基,也是其先前鉴定的异亮氨酸拉链序列的“ a”位,与在第19位的脯氨酸残基交换。结果,观察到BMAP-28对人红细胞(hRBCs)的溶血活性显着降低,而没有在液体介质中以及存在盐和血清的情况下抑制其抗菌活性。BMAP-28类似物,BMAP-28M去极化金黄色葡萄球菌膜和细菌膜模拟PE / PG脂质囊泡类似于BMAP-28,但显示出对hRBC膜和哺乳动物膜模拟PC / Chol囊泡去极化的功效较弱。仅在PC / Chol囊泡中观察到BMAP-28和BMAP-28M色氨酸残基的定位差异。BMAP-28在hRBC上诱导了直径约3.4 nm的孔,而BMAP-28M在这些细胞上显示出较小的扰动。BMAP-28和BMAP-28M诱导钙黄绿素(尺寸约1 nm)和4.4 kDa FITC-葡聚糖(尺寸约2.8 nm)进入巨大芽孢杆菌中,但未引入较大尺寸的荧光葡聚糖,表明在其上形成了环形孔细菌膜。











































 京公网安备 11010802027423号
京公网安备 11010802027423号