当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A validated method for the simultaneous quantification of cannabidiol, Δ9‐tetrahydrocannabinol, and their metabolites in human plasma and application to plasma samples from an oral cannabidiol open‐label trial
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-10-23 , DOI: 10.1002/dta.2947 Richard C Kevin 1, 2 , Rebecca Vogel 2, 3 , Peter Doohan 2 , Maximus Berger 4, 5 , G Paul Amminger 4, 5 , Iain S McGregor 1, 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-10-23 , DOI: 10.1002/dta.2947 Richard C Kevin 1, 2 , Rebecca Vogel 2, 3 , Peter Doohan 2 , Maximus Berger 4, 5 , G Paul Amminger 4, 5 , Iain S McGregor 1, 2
Affiliation
|
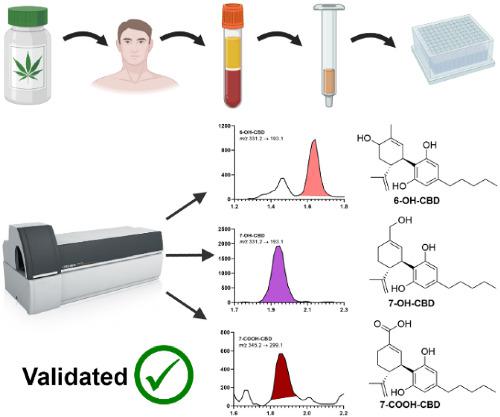
Cannabidiol (CBD) and Δ9‐tetrahydrocannabinol (THC) are the two best known and most extensively studied phytocannabinoids within Cannabis sativa. An increasing number of preclinical studies and clinical trials have been conducted with one or both compounds, often probing their therapeutic effects in conditions such as paediatric epilepsy, anxiety disorders or chronic pain. Accurate monitoring of THC and CBD and their metabolites is essential for tracking treatment adherence and pharmacokinetics. However, fully validated methods for the comprehensive analysis of major Phase I CBD metabolites are yet to be developed due to a historical lack of commercially available reference material. In the present study, we developed, optimised and validated a method for the simultaneous quantification of CBD, THC and their major Phase I metabolites 6‐hydroxy‐CBD (6‐OH‐CBD), 7‐hydroxy‐CBD (7‐OH‐CBD), 7‐carboxy‐CBD (7‐COOH‐CBD), 11‐hydroxy‐tetrahydrocannabinol (11‐OH‐THC) and 11‐carboxy‐tetrahydrocannabinol (11‐COOH‐THC) as per Food and Drug Administration (FDA) guidelines for bioanalytical method validation. The method is accurate, reproducible, sensitive and can be carried out in high‐throughput 96‐well formats, ideal for larger scale clinical trials. Deuterated internal standards for each analyte were crucial to account for variable matrix effects between plasma lots. The application of the method to plasma samples, taken from people who had been administered oral CBD as part of an open‐label trial of CBD effects in anxiety disorders, demonstrated its immediate utility in ongoing and upcoming clinical trials. The method will prove useful for future studies involving CBD and/or THC and can likely accommodate the inclusion of additional metabolites as analytical reference materials become commercially available.
中文翻译:

一种经验证的方法,用于同时定量人血浆中的大麻二酚、Δ9-四氢大麻酚及其代谢物,并应用于口服大麻二酚开放标签试验的血浆样品
大麻二酚(CBD)和Δ 9 -四氢大麻酚(THC)是内的两个最有名的和最广泛研究的phytocannabinoids大麻. 越来越多的临床前研究和临床试验是用一种或两种化合物进行的,经常探索它们在小儿癫痫、焦虑症或慢性疼痛等疾病中的治疗效果。准确监测 THC 和 CBD 及其代谢物对于跟踪治疗依从性和药代动力学至关重要。然而,由于历史上缺乏市售参考材料,尚未开发出对主要 I 期 CBD 代谢物进行全面分析的经过充分验证的方法。在本研究中,我们开发、优化和验证了一种同时定量 CBD、THC 及其主要 I 期代谢物 6-羟基-CBD (6-OH-CBD)、7-羟基-CBD (7-OH- CBD)、7-羧基-CBD (7-COOH-CBD)、11-羟基-四氢大麻酚 (11-OH-THC) 和 11-羧基-四氢大麻酚 (11-COOH-THC) 根据食品和药物管理局 (FDA) 生物分析方法验证指南。该方法准确、可重复、灵敏,可以在高通量 96 孔格式下进行,非常适合大规模临床试验。每种分析物的氘代内标对于解释血浆批次之间的可变基质效应至关重要。将该方法应用于血浆样本,这些样本取自口服 CBD 的人,作为 CBD 对焦虑症影响的开放标签试验的一部分,证明了其在正在进行和即将进行的临床试验中的直接效用。
更新日期:2020-10-23
中文翻译:

一种经验证的方法,用于同时定量人血浆中的大麻二酚、Δ9-四氢大麻酚及其代谢物,并应用于口服大麻二酚开放标签试验的血浆样品
大麻二酚(CBD)和Δ 9 -四氢大麻酚(THC)是内的两个最有名的和最广泛研究的phytocannabinoids大麻. 越来越多的临床前研究和临床试验是用一种或两种化合物进行的,经常探索它们在小儿癫痫、焦虑症或慢性疼痛等疾病中的治疗效果。准确监测 THC 和 CBD 及其代谢物对于跟踪治疗依从性和药代动力学至关重要。然而,由于历史上缺乏市售参考材料,尚未开发出对主要 I 期 CBD 代谢物进行全面分析的经过充分验证的方法。在本研究中,我们开发、优化和验证了一种同时定量 CBD、THC 及其主要 I 期代谢物 6-羟基-CBD (6-OH-CBD)、7-羟基-CBD (7-OH- CBD)、7-羧基-CBD (7-COOH-CBD)、11-羟基-四氢大麻酚 (11-OH-THC) 和 11-羧基-四氢大麻酚 (11-COOH-THC) 根据食品和药物管理局 (FDA) 生物分析方法验证指南。该方法准确、可重复、灵敏,可以在高通量 96 孔格式下进行,非常适合大规模临床试验。每种分析物的氘代内标对于解释血浆批次之间的可变基质效应至关重要。将该方法应用于血浆样本,这些样本取自口服 CBD 的人,作为 CBD 对焦虑症影响的开放标签试验的一部分,证明了其在正在进行和即将进行的临床试验中的直接效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号