当前位置:
X-MOL 学术
›
Neuropathol. Appl. Neurobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IDH Clonal Heterogeneity Segregates a Subgroup of Non‐1p/19q Codeleted Gliomas with Unfavourable Clinical Outcome
Neuropathology and Applied Neurobiology ( IF 4.0 ) Pub Date : 2020-11-12 , DOI: 10.1111/nan.12671 Shangyi Luo 1 , Shiwei Zhu 1 , Jianlong Liao 1 , Yajing Zhang 2 , Xiaobo Hou 1 , Tao Luo 1 , Erjie Zhao 1 , Jinyuan Xu 1 , Lin Pang 1 , Xin Liang 1 , Yun Xiao 1 , Xia Li 1
Neuropathology and Applied Neurobiology ( IF 4.0 ) Pub Date : 2020-11-12 , DOI: 10.1111/nan.12671 Shangyi Luo 1 , Shiwei Zhu 1 , Jianlong Liao 1 , Yajing Zhang 2 , Xiaobo Hou 1 , Tao Luo 1 , Erjie Zhao 1 , Jinyuan Xu 1 , Lin Pang 1 , Xin Liang 1 , Yun Xiao 1 , Xia Li 1
Affiliation
|
AIMS
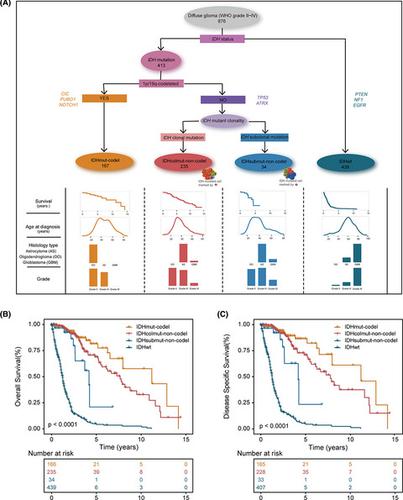
Diffuse gliomas (DGs) are classified into three major molecular subgroups following the revised World Health Organisation (WHO) classification criteria based on their IDH mutation and 1p/19q codeletion status. However, substantial biological heterogeneity and differences in the clinical course are apparent within each subgroup, which remain to be resolved. We sought to assess the clonal status of somatic mutations and explore whether additional molecular subgroups exist within DG. METHODS
A computational framework that integrates the variant allele frequency, local copy number and tumour purity was used to infer the clonality of somatic mutations in 876 DGs from The Cancer Genome Atlas (TCGA). We performed an unsupervised cluster analysis to identify molecular subgroups and characterised their clinical and biological significance. RESULTS
DGs showed widespread genetic intratumoural heterogeneity (ITH), with nearly all driver genes harbouring subclonal mutations, even for known glioma initiating event IDH1 (17.1%). Gliomas with subclonal IDH mutation and without 1p/19q codeletion showed shorter overall and disease specific survival, higher ITH, and exhibited differences in genomic patterns, transcript levels and proliferative potential, when compared with IDH clonal mutation and no 1p/19q codeletion gliomas. We defined a refined stratification system based on the current WHO glioma molecular classification, which showed close correlations with patients' clinical outcomes. CONCLUSIONS
For the first time, we integrated the clonal status of somatic mutations into cancer genomic classification and highlighted the necessity of considering IDH clonal architectures in glioma precision stratification.
中文翻译:

IDH 克隆异质性分离了具有不利临床结果的非 1p/19q 编码神经胶质瘤亚组
AIMS 根据其 IDH 突变和 1p/19q 编码缺失状态,按照修订后的世界卫生组织 (WHO) 分类标准将弥漫性胶质瘤 (DG) 分为三个主要分子亚组。然而,在每个亚组中,明显的生物学异质性和临床病程的差异仍有待解决。我们试图评估体细胞突变的克隆状态,并探讨 DG 中是否存在其他分子亚群。方法 使用整合变异等位基因频率、局部拷贝数和肿瘤纯度的计算框架来推断来自癌症基因组图谱 (TCGA) 的 876 个 DG 中体细胞突变的克隆性。我们进行了无监督的聚类分析,以确定分子亚群并表征它们的临床和生物学意义。结果 DG 显示出广泛的遗传肿瘤内异质性 (ITH),几乎所有驱动基因都包含亚克隆突变,即使是已知的胶质瘤起始事件 IDH1 (17.1%)。与 IDH 克隆突变且无 1p/19q 共缺失的胶质瘤相比,具有亚克隆 IDH 突变且无 1p/19q 共缺失的胶质瘤显示出较短的总体和疾病特异性生存期、更高的 ITH,并在基因组模式、转录水平和增殖潜力方面表现出差异。我们根据当前的 WHO 神经胶质瘤分子分类定义了一个精细的分层系统,该系统与患者的临床结果密切相关。结论 第一次,
更新日期:2020-11-12
中文翻译:

IDH 克隆异质性分离了具有不利临床结果的非 1p/19q 编码神经胶质瘤亚组
AIMS 根据其 IDH 突变和 1p/19q 编码缺失状态,按照修订后的世界卫生组织 (WHO) 分类标准将弥漫性胶质瘤 (DG) 分为三个主要分子亚组。然而,在每个亚组中,明显的生物学异质性和临床病程的差异仍有待解决。我们试图评估体细胞突变的克隆状态,并探讨 DG 中是否存在其他分子亚群。方法 使用整合变异等位基因频率、局部拷贝数和肿瘤纯度的计算框架来推断来自癌症基因组图谱 (TCGA) 的 876 个 DG 中体细胞突变的克隆性。我们进行了无监督的聚类分析,以确定分子亚群并表征它们的临床和生物学意义。结果 DG 显示出广泛的遗传肿瘤内异质性 (ITH),几乎所有驱动基因都包含亚克隆突变,即使是已知的胶质瘤起始事件 IDH1 (17.1%)。与 IDH 克隆突变且无 1p/19q 共缺失的胶质瘤相比,具有亚克隆 IDH 突变且无 1p/19q 共缺失的胶质瘤显示出较短的总体和疾病特异性生存期、更高的 ITH,并在基因组模式、转录水平和增殖潜力方面表现出差异。我们根据当前的 WHO 神经胶质瘤分子分类定义了一个精细的分层系统,该系统与患者的临床结果密切相关。结论 第一次,











































 京公网安备 11010802027423号
京公网安备 11010802027423号