当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of the Electrochemical Response of Surface‐confined Bidirectional Molecular Electrocatalysts in the Presence of Intermolecular Interactions
ChemCatChem ( IF 3.8 ) Pub Date : 2020-10-23 , DOI: 10.1002/cctc.202001599 Joaquín González 1 , José‐Alfonso Sequí 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-10-23 , DOI: 10.1002/cctc.202001599 Joaquín González 1 , José‐Alfonso Sequí 1
Affiliation
|
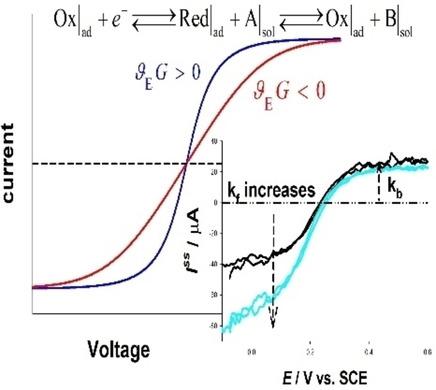
A theoretical model for the response of bidirectional surface confined molecular electrocatalysts in the presence of intermolecular interactions is presented. Particular cases corresponding to Nernstian and fully irreversible redox behaviour are discussed, with the main features of the stationary current‐potential response being analysed in detail. This analysis has revealed important nuances in the understanding of the overall process relative to the limits of validity of some frequent assumptions, as for example, a Nernstian behaviour of the charge transfer step and therefore of the complete catalytic mechanism. The presence of interactions leads to a distortion of the current‐potential responses for which, in the case of repulsive interactions, higher overpotential values are required for a given conversion rate. In the case of non‐Nernstian charge transfer processes, high catalytic rates give rise to an increase of the overall irreversibility of the catalytic currents. The experimental verification of the model corresponding to the response of binary 11‐(ferrocenyl)undecanethiol/decanethiol in presence of ferro/ferricyanide is presented and discussed. The impact of the experimental conditions on the apparent values of the formal potential and redox rate constants of the molecular electrocatalyst is also analysed.
中文翻译:

存在分子间相互作用的表面受限双向分子催化剂的电化学响应分析
提出了在分子间相互作用下双向表面受限分子电催化剂响应的理论模型。讨论了与Nernstian和完全不可逆的氧化还原行为相对应的特殊情况,并详细分析了稳定电流电位响应的主要特征。该分析表明,相对于一些常见假设的有效性限制,在理解整个过程方面存在重要的细微差别,例如,电荷转移步骤的Nernstian行为以及因此而形成的完整催化机制。相互作用的存在会导致电流电势响应的失真,在排斥性相互作用的情况下,对于给定的转换率,需要更高的超电势值。在非能斯特电荷转移过程中,高催化速率会增加催化电流的整体不可逆性。提出并讨论了模型的实验验证,该模型对应于二元11-(二茂铁基)十一烷硫醇/癸硫醇在铁/铁氰化物存在下的响应。还分析了实验条件对分子电催化剂的形式势和氧化还原速率常数的表观值的影响。
更新日期:2020-10-23
中文翻译:

存在分子间相互作用的表面受限双向分子催化剂的电化学响应分析
提出了在分子间相互作用下双向表面受限分子电催化剂响应的理论模型。讨论了与Nernstian和完全不可逆的氧化还原行为相对应的特殊情况,并详细分析了稳定电流电位响应的主要特征。该分析表明,相对于一些常见假设的有效性限制,在理解整个过程方面存在重要的细微差别,例如,电荷转移步骤的Nernstian行为以及因此而形成的完整催化机制。相互作用的存在会导致电流电势响应的失真,在排斥性相互作用的情况下,对于给定的转换率,需要更高的超电势值。在非能斯特电荷转移过程中,高催化速率会增加催化电流的整体不可逆性。提出并讨论了模型的实验验证,该模型对应于二元11-(二茂铁基)十一烷硫醇/癸硫醇在铁/铁氰化物存在下的响应。还分析了实验条件对分子电催化剂的形式势和氧化还原速率常数的表观值的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号