当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revelation of point mutations effect in Mycobacterium tuberculosis MfpA protein that involved in mycobacterial DNA supercoiling and fluoroquinolone resistance
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-10-23 , DOI: 10.1002/bab.2058 Princy Gautam 1 , Shivangi 1, 2 , Laxman S Meena 1, 2
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-10-23 , DOI: 10.1002/bab.2058 Princy Gautam 1 , Shivangi 1, 2 , Laxman S Meena 1, 2
Affiliation
|
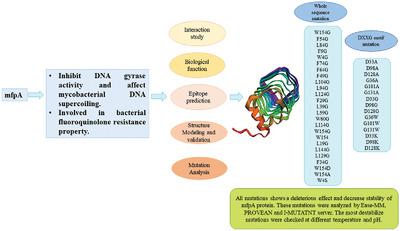
MfpA protein is encoded by Mycobacterium tuberculosis (Mtb) and stands for Mycobacterium fluoroquinolone resistance protein A. This protein provides Mtb intrinsic resistant property from fluoroquinolone antibiotics by inhibiting DNA gyrase that are known to be the primary target of fluoroquinolone drugs. DNA gyrases are important for bacterial chromosomal genesis as they are majorly involved in DNA replication, transcription, and bacterial stress response to several external stimulus. Therefore, in Mtb, it forms an essential integrity and also a desirable target for drug development approaches. This paper implies on determining the essential facts about mfpA including its interaction study, epitope prediction, modeling, and validation, and most importantly it deals with the mutation. Mutational analysis was carried out on the basis of sequential information and there were several mutations that cause a large decrease in the stability of the protein. Total 24 mutations were shortlisted based on ΔΔG value: W154G, F54G, L84G, F9G, W4G, F74G, F64G, F49G, L104G, L94G, L124G, F29G, L39G, L59G, W60G, L114G, , W154S, L19G, L144G, L129G, F34G, W154D, W154A, and W4S. Separate mutation on DXXG GTPase motif was examined to check any effect on protein stability and we found that D33A, D98A, D128A, G36A, G101A, G131A, D33G, D98G, D128G, G36W, G101W, G131W, D33K, D98K, and D128K decrease protein stability the most. Further stress-dependent analysis on selected residues showed that lower temperature and pH destabilize the protein. The reason behind this increase in protein destability was drastic decrease and disruption of interatomic interactions in mutant MfpA. These analysis provides essential information about the residues that are important for MfpA stability and also enlightens protein vulnerability after mutation.
中文翻译:

结核分枝杆菌MfpA蛋白点突变参与分枝杆菌DNA超螺旋和氟喹诺酮耐药的揭示
MfpA 蛋白由结核分枝杆菌( Mtb ) 编码,代表分枝杆菌氟喹诺酮耐药蛋白 A。该蛋白通过抑制 DNA 旋转酶(已知是氟喹诺酮类药物的主要靶标),为Mtb提供对氟喹诺酮类抗生素的固有耐药特性。 DNA 旋转酶对于细菌染色体发生很重要,因为它们主要参与 DNA 复制、转录和细菌对多种外部刺激的应激反应。因此,在Mtb中,它形成了一个基本的完整性,也是药物开发方法的理想目标。本文旨在确定 mfpA 的基本事实,包括其相互作用研究、表位预测、建模和验证,最重要的是它涉及突变。根据序列信息进行突变分析,有多个突变导致蛋白质稳定性大幅下降。根据ΔΔG值入围总共24个突变:W154G、F54G、L84G、F9G、W4G、F74G、F64G、F49G、L104G、L94G、L124G、F29G、L39G、L59G、W60G、L114G、、W154S、L19G、L144G、 L129G、F34G、W154D、W154A 和 W4S。检查 DXXG GTPase 基序的单独突变以检查对蛋白质稳定性的影响,我们发现 D33A、D98A、D128A、G36A、G101A、G131A、D33G、D98G、D128G、G36W、G101W、G131W、D33K、D98K 和 D128K 减少蛋白质稳定性最强。对选定残基的进一步压力依赖性分析表明,较低的温度和 pH 值会使蛋白质不稳定。蛋白质不稳定性增加的原因是突变体 MfpA 中原子间相互作用的急剧减少和破坏。 这些分析提供了有关对 MfpA 稳定性很重要的残基的基本信息,也揭示了突变后蛋白质的脆弱性。
更新日期:2020-10-23
中文翻译:

结核分枝杆菌MfpA蛋白点突变参与分枝杆菌DNA超螺旋和氟喹诺酮耐药的揭示
MfpA 蛋白由结核分枝杆菌( Mtb ) 编码,代表分枝杆菌氟喹诺酮耐药蛋白 A。该蛋白通过抑制 DNA 旋转酶(已知是氟喹诺酮类药物的主要靶标),为Mtb提供对氟喹诺酮类抗生素的固有耐药特性。 DNA 旋转酶对于细菌染色体发生很重要,因为它们主要参与 DNA 复制、转录和细菌对多种外部刺激的应激反应。因此,在Mtb中,它形成了一个基本的完整性,也是药物开发方法的理想目标。本文旨在确定 mfpA 的基本事实,包括其相互作用研究、表位预测、建模和验证,最重要的是它涉及突变。根据序列信息进行突变分析,有多个突变导致蛋白质稳定性大幅下降。根据ΔΔG值入围总共24个突变:W154G、F54G、L84G、F9G、W4G、F74G、F64G、F49G、L104G、L94G、L124G、F29G、L39G、L59G、W60G、L114G、、W154S、L19G、L144G、 L129G、F34G、W154D、W154A 和 W4S。检查 DXXG GTPase 基序的单独突变以检查对蛋白质稳定性的影响,我们发现 D33A、D98A、D128A、G36A、G101A、G131A、D33G、D98G、D128G、G36W、G101W、G131W、D33K、D98K 和 D128K 减少蛋白质稳定性最强。对选定残基的进一步压力依赖性分析表明,较低的温度和 pH 值会使蛋白质不稳定。蛋白质不稳定性增加的原因是突变体 MfpA 中原子间相互作用的急剧减少和破坏。 这些分析提供了有关对 MfpA 稳定性很重要的残基的基本信息,也揭示了突变后蛋白质的脆弱性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号