当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B/N-Doped p-Arylenevinylene Chromophores: Synthesis, Properties, and Microcrystal Electron Crystallographic Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-22 , DOI: 10.1021/jacs.0c10337 Hua Lu 1, 2 , Takayuki Nakamuro 1 , Keitaro Yamashita 3 , Haruaki Yanagisawa 4 , Osamu Nureki 3 , Masahide Kikkawa 4 , Han Gao 5 , Jiangwei Tian 5 , Rui Shang 1 , Eiichi Nakamura 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-22 , DOI: 10.1021/jacs.0c10337 Hua Lu 1, 2 , Takayuki Nakamuro 1 , Keitaro Yamashita 3 , Haruaki Yanagisawa 4 , Osamu Nureki 3 , Masahide Kikkawa 4 , Han Gao 5 , Jiangwei Tian 5 , Rui Shang 1 , Eiichi Nakamura 1
Affiliation
|
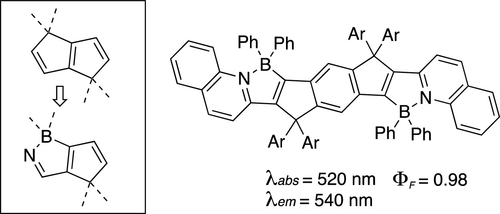
Linearly conjugated systems have long served as an archetype of conjugated materials, but suffer from two intrinsic structural problems: potential instability due to intermolecular interactions and the flexibility of the C-C bonds connecting C═C bonds. Efforts to solve these problems have included the insertion of aromatic units as a part of the conjugation and the introduction of carbon bridges to stop the bond rotation. We report here B/N-doped p-arylenevinylene chromophores synthesized through the incorporation of a cyclopenta[c][1,2]azaborole framework as a part of the conjugated system. The ring strain intrinsic to this new skeleton both flattens and rigidifies the conjugation, and the B--N+ dative bond is much easier to form than a C-C bond, which simplifies the synthetic design. The B-N dative bond also reduces the HOMO-LUMO gap, thereby causing a significant redshift of the absorption and emission compared with their all-carbon congeners while retaining high photostability and high fluorescence quantum yield in both solution and film states. A doubly B/N-doped compound showed emission peaks at 540 nm with a small Stokes shift of 20 nm and a fluorescence quantum yield of 98%. The molecules serve as excellent lipophilic fluorescent dyes for live-cell imaging, showing a higher photostability than that of commercially available BODIPY-based dyes.
中文翻译:

B/N 掺杂对亚芳基亚乙烯基发色团:合成、性质和微晶电子晶体学研究
线性共轭体系长期以来一直是共轭材料的原型,但存在两个固有的结构问题:分子间相互作用导致的潜在不稳定性和连接 C=C 键的 CC 键的灵活性。解决这些问题的努力包括插入芳香单元作为共轭的一部分和引入碳桥以阻止键旋转。我们在此报告了通过将环戊二烯 [c][1,2] 氮杂硼骨架作为共轭系统的一部分并入合成的 B/N 掺杂对亚芳基亚乙烯基发色团。这种新骨架固有的环应变既使共轭变平,又使共轭硬化,并且 B--N+ 配键比 CC 键更容易形成,这简化了合成设计。BN 配键也减少了 HOMO-LUMO 间隙,因此,与它们的全碳同系物相比,吸收和发射发生显着红移,同时在溶液和薄膜状态下保持高光稳定性和高荧光量子产率。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂荧光染料,表现出比市售 BODIPY 染料更高的光稳定性。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂性荧光染料,显示出比市售 BODIPY 染料更高的光稳定性。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂性荧光染料,显示出比市售 BODIPY 染料更高的光稳定性。
更新日期:2020-10-22
中文翻译:

B/N 掺杂对亚芳基亚乙烯基发色团:合成、性质和微晶电子晶体学研究
线性共轭体系长期以来一直是共轭材料的原型,但存在两个固有的结构问题:分子间相互作用导致的潜在不稳定性和连接 C=C 键的 CC 键的灵活性。解决这些问题的努力包括插入芳香单元作为共轭的一部分和引入碳桥以阻止键旋转。我们在此报告了通过将环戊二烯 [c][1,2] 氮杂硼骨架作为共轭系统的一部分并入合成的 B/N 掺杂对亚芳基亚乙烯基发色团。这种新骨架固有的环应变既使共轭变平,又使共轭硬化,并且 B--N+ 配键比 CC 键更容易形成,这简化了合成设计。BN 配键也减少了 HOMO-LUMO 间隙,因此,与它们的全碳同系物相比,吸收和发射发生显着红移,同时在溶液和薄膜状态下保持高光稳定性和高荧光量子产率。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂荧光染料,表现出比市售 BODIPY 染料更高的光稳定性。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂性荧光染料,显示出比市售 BODIPY 染料更高的光稳定性。双 B/N 掺杂化合物在 540 nm 处显示发射峰,斯托克斯位移为 20 nm,荧光量子产率为 98%。这些分子可作为用于活细胞成像的优良亲脂性荧光染料,显示出比市售 BODIPY 染料更高的光稳定性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号