当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enabling Facile Anionic Kinetics through Cationic Redox Mediator in Li-Rich Layered Cathodes
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsenergylett.0c01880 Ning Li 1 , Jue Wu 2, 3 , Sooyeon Hwang 4 , Joseph K. Papp 5 , Wang Hay Kan 6 , Liang Zhang 2 , Chenhui Zhu 2 , Bryan D. McCloskey 1, 5 , Wanli Yang 2 , Wei Tong 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsenergylett.0c01880 Ning Li 1 , Jue Wu 2, 3 , Sooyeon Hwang 4 , Joseph K. Papp 5 , Wang Hay Kan 6 , Liang Zhang 2 , Chenhui Zhu 2 , Bryan D. McCloskey 1, 5 , Wanli Yang 2 , Wei Tong 1
Affiliation
|
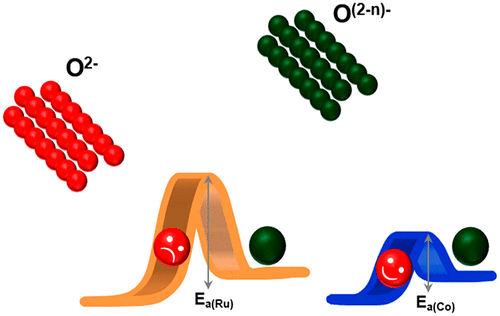
Anionic oxygen redox has aroused great interest in developing high-capacity Li-ion battery cathode materials. The fundamental understanding of this concept, compared to cationic redox, has promoted extensive studies on lithium transition metal oxides including those of 4d and 5d transition metals. Lithium ruthenium oxide has been found to exhibit a reversible anionic redox upon cycling. However, lithium-rich layered oxide with anionic redox is still facing great challenges, such as sluggish kinetics. Here we investigate the effect of cationic redox reaction on the kinetics of anionic reaction when they are strongly coupled. We report the cobalt-substituted lithium ruthenium oxide, where all Ru, Co, and O redox participate in the charge compensation mechanism in relatively defined voltage regions. The improved anionic kinetics is attributed to the fast cationic Co redox process that serves as a redox mediator. Our work sheds light on the potential direction to address the commonly believed sluggish anionic kinetics in high-capacity oxygen-redox cathode materials.
中文翻译:

通过富Li层状阴极中的阳离子氧化还原介体实现简便的阴离子动力学
阴离子氧氧化还原引起了人们对开发高容量锂离子电池正极材料的极大兴趣。与阳离子氧化还原相比,对该概念的基本理解促进了对锂过渡金属氧化物(包括4d和5d过渡金属的氧化物)的广泛研究。已经发现锂钌氧化物在循环时表现出可逆的阴离子氧化还原。然而,具有阴离子氧化还原的富含锂的层状氧化物仍面临巨大的挑战,例如动力学迟缓。在这里,我们研究了阳离子氧化还原反应对强耦合的阴离子反应动力学的影响。我们报告了钴取代的锂钌氧化物,其中所有Ru,Co和O氧化还原都在相对定义的电压区域中参与电荷补偿机制。改善的阴离子动力学归因于充当氧化还原介质的快速阳离子Co氧化还原过程。我们的工作阐明了解决高容量氧-氧化还原阴极材料中通常被认为缓慢的阴离子动力学的潜在方向。
更新日期:2020-11-13
中文翻译:

通过富Li层状阴极中的阳离子氧化还原介体实现简便的阴离子动力学
阴离子氧氧化还原引起了人们对开发高容量锂离子电池正极材料的极大兴趣。与阳离子氧化还原相比,对该概念的基本理解促进了对锂过渡金属氧化物(包括4d和5d过渡金属的氧化物)的广泛研究。已经发现锂钌氧化物在循环时表现出可逆的阴离子氧化还原。然而,具有阴离子氧化还原的富含锂的层状氧化物仍面临巨大的挑战,例如动力学迟缓。在这里,我们研究了阳离子氧化还原反应对强耦合的阴离子反应动力学的影响。我们报告了钴取代的锂钌氧化物,其中所有Ru,Co和O氧化还原都在相对定义的电压区域中参与电荷补偿机制。改善的阴离子动力学归因于充当氧化还原介质的快速阳离子Co氧化还原过程。我们的工作阐明了解决高容量氧-氧化还原阴极材料中通常被认为缓慢的阴离子动力学的潜在方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号